Abstract
Background:
Hematopoietic stem cell transplantation (HSCT) is cure for thalassemia major (TM). However, a suitable donor (HLA matched sibling and unrelated donor) for HSCT is less than 50%. Alternative donors were recently used in TM HSCT. Some study have found that thalassemia-free survival (TFS) was approximately 70% in haploidentical HSCT (h-HSCT) or unrelated cord blood (UCB) transplant for TM patients. So, it is necessary to find out a better h-HSCT for TM patients. In our early practice in leukemic HSCT we found that outcomes were improved by adding UCB to post-transplant cyclophosphamide (PT/Cy) h-HSCT. The latter associated with high mortality related transplant (32%). Henceforth, we used this termed haplocord transplant in TM.
Aim:
To develop a high TFS h-HSCT protocol for TM patients.
Patients and methods
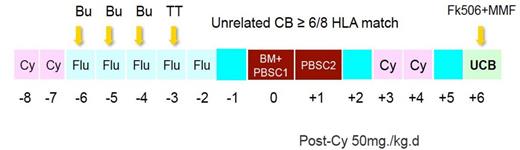
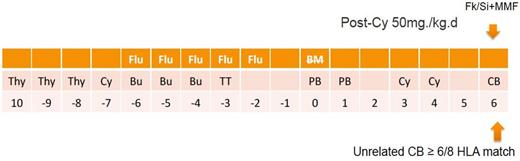
First 10 patients with median age 8 (5-17) old years received NF-13-PT/Cy-TM protocol (fig. 1), in which, UCB was added on day 6 after PT/CY h-HSCT. Following 9 patients with age 9 (4-15) old years received NF-14-PT/Cy-TM protocol (fig. 2), in which three doses Thymoglobuline were added to NF-13-PT/Cy-TM protocol. Cyclophosphamide on day 3 and day 4 after transplant were both GVHD prophylaxis for h-HSCT and conditioning for UCB transplant. The HLA (at HLA-A, -B, -C and ¨CDRB1) for the pair of recipient and donor was 2-loci and more mismatched in h-HSCT and 2-loci and less mismatched in UCB.
Results
The results of haplocord transplants for all patients were showed in table 1. For first 10 patients, final cord blood engrafted in 4 patients; final haploidentical donor engrafted in 3 patients, 2 patients had a primary rejection. One had a secondary rejection and gave up therapy and died of infection. One patient died of grade IV acute GVHD. TFS is 6/10. For second group patients, final cord blood engrafted in 4 patients; final haploidentical donor engrafted in 3 patients, mixed donor engrafted in 2. No patient rejected his graft; All 9 patients live with transfusion independence.
Summary
Our data showed that UCB followed PT/Cy h-HSCT using NF-14-PT/Cy-TM protocol improved the results of alternative donor transplant in thalassemia major.
Registered in Clinical Trials: NCT02126046,
| Case . | Gender /Age(Y) . | Transplant Time . | Months After Transplantation . | Last engraftment (Month) . | Current Status . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | |||||
| 1 | M/8 | 2012.09 | Mix | Mix | Mix | Mix | Mix | Mix | CB (15) | Alive |
| 2 | M/5 | 2012.11 | CB | CB | CB | CB | CB | CB | CB | Alive |
| 3 | M/6 | 2013.01 | / | / | / | / | / | / | / | Reject |
| 4 | M/17 | 2013.03 | PB | PB | PB | PB | PB | PB | PB | Alive |
| 5 | M/11 | 2013.11 | Mix | Mix | Mix | Mix | Mix | Mix | Mix (14) | Dead |
| 6 | M/6 | 2013.12 | Mix | Mix | Mix | CB | CB | CB | CB | Alive |
| 7 | F/17 | 2014.03 | Mix | Mix | Mix | CB | CB | CB | CB | Alive |
| 8 | F/7 | 2014.05 | PB | PB | PB | PB | PB | PB | PB | Alive |
| 9 | F/14 | 2014.05 | PB | PB | PB | Dead | / | / | PB (3) | Dead |
| 10 | M/8 | 2014.05 | / | / | / | / | / | / | / | Reject |
| 11 | M/9 | 2014.08 | Mix | PB | PB | PB | PB | PB | PB | Alive |
| 12 | M/9 | 2014.08 | Mix | PB | PB | Mix | Mix | Mix | PB (7) | Alive |
| 13 | M/9 | 2014.10 | Mix | Mix | Mix | Mix | Mix | Mix | Mix (9) | Alive |
| 14 | M/4 | 2014.10 | Mix | Mix | CB | CB | CB | CB | CB | Alive |
| 15 | F/7 | 2014.11 | PB | PB | PB | PB | PB | PB | PB | Alive |
| 16 | M/8 | 2014.12 | Mix | Mix | Mix | Mix | Mix | CB | CB | Alive |
| 17 | M/15 | 2014.12 | Mix | Mix | Mix | CB | CB | CB | CB | Alive |
| 18 | M/14 | 2015.03 | PB | PB | PB | PB | PB | Alive | ||
| 19 | F/14 | 2015.06 | Mix | CB | CB | Alive | ||||
| Case . | Gender /Age(Y) . | Transplant Time . | Months After Transplantation . | Last engraftment (Month) . | Current Status . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | |||||
| 1 | M/8 | 2012.09 | Mix | Mix | Mix | Mix | Mix | Mix | CB (15) | Alive |
| 2 | M/5 | 2012.11 | CB | CB | CB | CB | CB | CB | CB | Alive |
| 3 | M/6 | 2013.01 | / | / | / | / | / | / | / | Reject |
| 4 | M/17 | 2013.03 | PB | PB | PB | PB | PB | PB | PB | Alive |
| 5 | M/11 | 2013.11 | Mix | Mix | Mix | Mix | Mix | Mix | Mix (14) | Dead |
| 6 | M/6 | 2013.12 | Mix | Mix | Mix | CB | CB | CB | CB | Alive |
| 7 | F/17 | 2014.03 | Mix | Mix | Mix | CB | CB | CB | CB | Alive |
| 8 | F/7 | 2014.05 | PB | PB | PB | PB | PB | PB | PB | Alive |
| 9 | F/14 | 2014.05 | PB | PB | PB | Dead | / | / | PB (3) | Dead |
| 10 | M/8 | 2014.05 | / | / | / | / | / | / | / | Reject |
| 11 | M/9 | 2014.08 | Mix | PB | PB | PB | PB | PB | PB | Alive |
| 12 | M/9 | 2014.08 | Mix | PB | PB | Mix | Mix | Mix | PB (7) | Alive |
| 13 | M/9 | 2014.10 | Mix | Mix | Mix | Mix | Mix | Mix | Mix (9) | Alive |
| 14 | M/4 | 2014.10 | Mix | Mix | CB | CB | CB | CB | CB | Alive |
| 15 | F/7 | 2014.11 | PB | PB | PB | PB | PB | PB | PB | Alive |
| 16 | M/8 | 2014.12 | Mix | Mix | Mix | Mix | Mix | CB | CB | Alive |
| 17 | M/15 | 2014.12 | Mix | Mix | Mix | CB | CB | CB | CB | Alive |
| 18 | M/14 | 2015.03 | PB | PB | PB | PB | PB | Alive | ||
| 19 | F/14 | 2015.06 | Mix | CB | CB | Alive | ||||
PB: Haploidetical PBSC; CB: cord blood
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.