Abstract
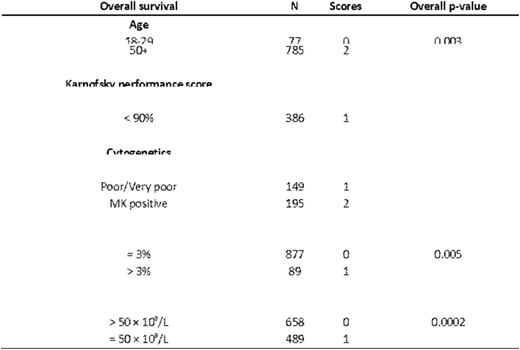
Allogeneic hematopoietic cell transplantation (allo HCT) is a curative therapy for myelodysplastic syndrome (MDS) that may result in toxicity and mortality, limiting its efficacy in patients with this disease. There is no standardized criterion to guide selection of patients with MDS for allo HCT. In order to address this problem we examined outcomes in 2,133 patients undergoing HLA matched or mismatched allo HCT for MDS reported to the Center for International Blood and Marrow Transplant Research from 2000-2012. The primary aim of this study is to develop a prognostic scoring system predictive of overall survival (OS) in this population. We additionally addressed whether the model is predictive of transplant related mortality (TRM), relapse, and disease free survival (DFS). Patients undergoing haploidentical, syngeneic, umbilical cord blood, or with missing donor data (N = 663) and pediatric patients (N = 262) were excluded from this analysis. An additional 84 patients were removed due to missing date of diagnosis, unknown graft versus host disease (GVHD) prophylaxis, or who were missing any 100-day follow up data. 1,728 patients met these criteria and underwent HLA-matched allo HCT. An additional 405 patients underwent HLA mismatched allo HCT and formed the HLA-mismatched set. The HLA-matched allo HCT set were randomly divided into a training data set comprising 67% (N = 1,151) of the cohort and a validation data set using the remaining 33% (N = 577). The training data set was used to develop a prognostic scoring system and the validation data set was used to assess the predictive ability of the scoring system. A Cox proportional hazards model with the stepwise selection procedure was used to select significant covariates for OS. Interactions between significant covariates were examined and proportional hazards assumption was examined. Based on the magnitude of the hazard ratios (HR) associated with variables a weighted score was assigned to factors that were positively associated with OS in the training cohort. Scores were grouped based on associated HRs into good, intermediate, high, and very high risk groups. Patients with missing data were included in the multivariate Cox model analysis but were excluded from the analysis of the final risk score in the training and validation set. This analysis identified five factors predictive of mortality in the HLA matched allo HCT training set: Peripheral blood blasts ≥ 3% or platelet count < 50 × 109/μL at transplantation, IPSS-R cytogenetic risk score, poor Karnofsky performance status, and older age at transplantation (Table 1). Using these variables we developed a MDS prognostic score (Table 2). We then used the scoring system defined in Table 2 to calculate a risk score for individuals in the training cohort that had complete data on all five variables (N = 839). Increasing score was associated with greater HR for death (p-overall < 0.0001). Based on these data we applied the score to the HLA-matched validation cohort, where increasing score was predictive of overall survival (p < 0.001). Because the training set was developed based on OS and not other outcomes, we combined the 839 cases from the training cohort with the 427 cases from the validation cohort for the analyses of the secondary endpoints. In the combined HLA-matched cohort the scoring system was associated with relapse (p < 0.0001), TRM (p < 0.0001), and DFS (p < 0.0001). We then tested this model in an additional set of individuals undergoing HLA-mismatched allo HCT (N = 405). Here, the score was predictive of relapse (p < 0.0001) but not OS, DFS, or TRM. In order to determine if the proposed scoring system is superior to the IPSS or IPSS-R prognostic tools we compared the three scoring systems in the HLA-matched validation set using concordance probabilities and Brier scores in 384 patients that had complete data for all three prognostic systems. The proposed scoring system was more predictive of OS when compared to the IPSS and IPSS-R using Brier (0.241, 0.252, and 0.249, respectively) and concordance probability tools (0.575, 0.538, and 0.554, respectively). In summary, we propose a system for prediction of outcomes in transplant recipients for MDS. Such a tool may be used to inform clinical decisions and to standardize mortality risk index in clinical trials examining transplantations in this patient population.
Maziarz:Athersys: Consultancy, Patents & Royalties, Research Funding; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.