Abstract
Chronic Graft Versus Host Disease (CGVHD) remains the main source of non-relapse mortality and morbidity among recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although our lab and others have identified infiltrates of Th1/Tc1 and Th17 effectors in skin and oral mucosa, CGVHD targets multiple organs and no common factor or pathway has been demonstrated to reflect the broad range of CGVHD inflammatory and fibrotic manifestations. To identify the systemic cytokine pathways supporting the development and persistence of CGVHD, we chose to profile gene expression in circulating monocytes; monocytes up-regulate distinct patterns of gene expression in response to different cytokines, acting as in situ reporters.
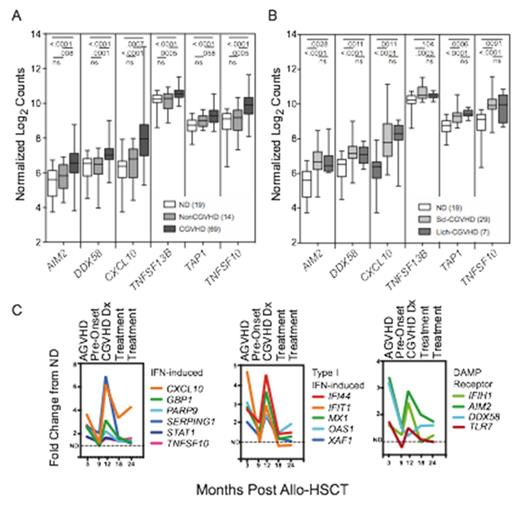
The NIH Natural History Study of CGVHD (NCT00092235) has primarily enrolled moderate to severely affected patients. Microarray analysis was performed on sorted monocytes from 10 normal controls (ND) and 26 patients selected from this cohort based on severe cutaneous involvement. Two interrelated pathways, each containing multiple genes, were consistently up-regulated across a cutaneous CGHVD spectrum ranging from lichenoid infiltrates to extensive sclerosis: (1) Interferon (IFN)-inducible genes including those involved in signaling, lymphocyte homeostasis and trafficking, apoptosis and antigen uptake and presentation (STAT1, CXCL10, TNFSF13B, TNFSF10, TAP1), and (2) innate immune receptors for pathogens and cellular damage that can trigger IFN production and inflammasome assembly (TLR2, TLR4, TLR7, AIM2, DDX58, CLEC4E). Using multiplex RNA gene expression assays (Nanostring) to verify these pathways, we found significant up-regulation of IFN-inducible and damage-response genes in 69 CGVHD patients with a broad range of organ involvement, as compared with 14 allo-HSCT patients never developing CGVHD, or with 19 normal controls (Figure 1A, B). These pathways were further substantiated in plasma ELISA assays showing elevated levels of IFN-induced chemokines (CXCL9, CXCL10) in both lichenoid and severe sclerotic patients. Immunohistochemistry substantiated expression of Type I IFN-induced factors (MxA) in inflammatory infiltrates in CGVHD-targeted organs: lichenoid and sclerotic skin, oral mucosa and salivary gland. Consistent with induction of Type I IFN by activation of TLR and RIG-I receptors, levels of expression of DDX58 and TLR7 correlated with up-regulation of Type I IFN inducible genes (OAS1, IFIT1, XAF1). Finally, multiplex RNA assessments on monocytes collected from 18 patients over serial time courses following NCI allo-HSCT protocols (NCT00520130 and NCT00074490) substantiated a pattern of parallel up-regulation of multiple IFN-inducible and damage responsive genes at CGVHD onset, and of decline upon therapy and resolution (Figure 1C). A key point is that comparable up-regulation of these pathways was found in patients with extensive lichenoid or sclerotic CGVHD, both in the established CGVHD patients in the initial microarray and in the serial time courses of CGVHD development. These results support a model that IFN and inflammasome activation induced by the innate immune systemÕs response to damage initiates an inflammatory process in CGVHD; IFN then can induce damage receptors, chemokines, cytokines and enhanced antigen presentation that sustain CGVHD. These interlocking analyses of gene expression patterns, plasma analytes and tissue are the first to support a unifying hypothesis of induction of IFN by innate response to cellular damage as a mechanism for initiation and persistence of CGVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.