Key Points
RIAM is an essential regulator of β2 integrins on leukocytes.
Leukocyte α4β1 integrin is activated in a RIAM-independent manner.
Abstract
Talin is an integrin adaptor, which controls integrin activity in all hematopoietic cells. How intracellular signals promote talin binding to the integrin tail leading to integrin activation is still poorly understood, especially in leukocytes. In vitro studies identified an integrin activation complex whose formation is initiated by the interaction of active, guanosine triphosphate (GTP)-bound Ras-related protein 1 (Rap1) with the adapter protein Rap1-GTP–interacting adapter molecule (RIAM) followed by the recruitment of talin to the plasma membrane. Unexpectedly, loss-of-function studies in mice have shown that the talin-activating role of RIAM is neither required for development nor for integrin activation in platelets. In this study, we show that leukocyte integrin activation critically depends on RIAM both in vitro and in vivo. RIAM deficiency results in a loss of β2 integrin activation in multiple leukocyte populations, impaired leukocyte adhesion to inflamed vessels, and accumulation in the circulation. Surprisingly, however, the major leukocyte β1 integrin family member, α4β1, was only partially affected by RIAM deficiency in leukocytes. Thus, although talin is an essential, shared regulator of all integrin classes expressed by leukocytes, we report that β2 and α4 integrins use different RIAM-dependent and -independent pathways to undergo activation by talin.
Introduction
Integrins are α/β heterodimeric transmembrane receptors that are expressed on all cells. They provide stable adhesion of cells to the extracellular matrix or to other cell counter receptors by forming complex adhesion sites, which serve as signaling platforms (outside-in signaling) that induce cell polarity, migration, survival, and proliferation. Because integrins reside in an inactive state, integrin-ligand binding requires an integrin activation step (inside-out signaling), which switches the heterodimer from a low- to a high-affinity state. The majority of studies that defined integrin affinity regulation were performed on αIIbβ3 and β2-class integrins expressed by platelets and leukocytes, respectively. These cells circulate in the blood and keep their integrins in an inactive state until they encounter soluble or membrane-bound agonists, which activate talin-1 and the hematopoietic cell-specific kindlin-3, which in turn cooperate to induce integrin activation and clustering.1,2 Consequently, loss of kindlin-3 or talin-1 expression in platelets and leukocytes abolishes activation of αIIbβ3 and β2 integrins leading to severe bleeding and leukocyte adhesion defects.3-6
Talin, which consists of an N-terminal band 4.1, ezrin, radixin, moesin (FERM) and a C-terminal rod domain, exists in an autoinhibited globular and active extended conformation that binds and activates integrins. In the autoinhibited state, the rod domain interacts with the FERM domain, which masks the integrin-binding site.7 This inhibition is released by binding of the membrane phospholipid phosphatidyl-inositol 4,5-bisphosphate (PIP2) to the talin head, which disrupts the autoinhibitory interaction between the rod domain and integrin binding region on the talin head and orients talin on the plasma membrane enabling β integrin tail binding.7-13
Talin activity has been shown to be regulated by activation of the small GTPase Ras-related protein 1 (Rap1), a known regulator of cell adhesion.14 Rap1-guanosine triphosphate (GTP)–interacting adapter molecule (RIAM) is a Rap1 effector molecule, which localizes to the leading edge of spreading cells and is expressed in many tissues. It belongs to the Mig-10/RIAM/lamellipodin (MRL) protein family, a group of multidomain adapter proteins involved in the regulation of actin dynamics, cell adhesion and migration, and cell growth.15 Knockdown of RIAM abrogated Rap1-induced adhesion to integrin ligands, whereas RIAM overexpression increased cell spreading and adhesion to β1 and β2 integrin ligands.16,17 RIAM was identified in signaling complexes downstream of the T-cell receptor and CC chemokine receptor 7, suggesting a role in integrin αLβ2 inside-out signaling.17,18 The molecular mechanism that underlies RIAM-mediated regulation of integrin activity was then further elaborated in a cell model system, which allowed reconstructing the molecular pathways resulting in the activation of the platelet integrin αIIbβ3. These studies proposed an integrin activation complex consisting of Rap1, RIAM, and talin. The prevailing view is that GTP-Rap1–bound RIAM recruits talin to the plasma membrane by binding the rod domain and subsequently activates talin by binding the integrin-binding region on the talin head.19-22 It has been postulated that the activation of talin by the GTP-Rap1/RIAM complex primarily serves to induce the formation of small, nascent adhesions in lamellipodia of spreading cells and that vinculin, which binds multiple sites on the talin rod, stabilizes the active conformation of talin, enabling the maturation of large focal adhesion.23,24
Despite overwhelming in vitro evidence for an important role of the ternary Rap1/RIAM/talin complex in integrin activation, genetic deletion of the RIAM gene in mice did not affect development, postnatal homeostasis, or platelet integrin functions.25 This unexpected outcome challenged the view of RIAM as critical integrin regulator. In this study, we demonstrate that an independently generated RIAM knockout mouse strain also showed no obvious phenotype, which is consistent with the report by Stritt et al.25 However, our analyses showed that RIAM plays an essential role in the activation of β2 integrins in neutrophils, macrophages, and T cells, resulting in a profound leukocyte adhesion defect. By contrast, β3 and β1 integrin functions remained normal or were only partially affected by the absence of RIAM in these leukocytes. Thus, our study highlights a novel specialized role of RIAM in activating β2 integrins and strongly suggests the existence of alternative RIAM-independent mechanisms for talin-mediated β1 and β3 integrin activation shared by both platelets and multiple types of leukocytes.
Materials and methods
Mice
RIAM−/− mice were generated by intercrossing mice, whose exon 4 of the RIAM gene was flanked with loxP sites (F.B.G., manuscript in preparation), with a deletor-Cre mouse. Talin-1fl/fl mice,3 β2 integrin−/− mice,26 CD4-Cre transgenic mice,27 and Mx1-Cre transgene mice28 have been described previously. All mice were from a mixed 129/SvJ × C57BL/6 background. Cre expression in the hematopoietic system was induced by intraperitoneal injection of 250 mg of poly-I/C (GE Healthcare). Around 4 × 106 bone marrow (BM) cells from wild-type (wt) or RIAM−/− mice were injected into the tail vein of lethally irradiated (10 Gy) RIAM−/− or wt recipient mice. Experiments with BM chimeras were performed 6 to 8 weeks after transfer. All animals were kept under specific pathogen-free conditions at the animal facility of the Max Planck Institute of Biochemistry.
All mouse experiments were approved by the district government of Bavaria.
Statistical analysis
Data are presented as means ± standard deviation (SD; in vitro data) or means ± standard error of the mean (SEM; in vivo and ex vivo data). Unpaired Student t tests (in vitro data) or 1-way analysis of variance followed by a multiple pairwise comparison test (Dunn test; in vivo and ex vivo data) were used to compare data sets.
Supplementary methods
Details on antibodies and in vitro and in vivo experiments are described in the supplemental Methods (available on the Blood Web site).
Results
RIAM is essential for β2 integrins on neutrophils
To study the role of RIAM in integrin regulation in vivo, we generated RIAM-deficient mice (RIAM−/−). RIAM heterozygous mice (RIAM+/−) expressed ∼50% of RIAM protein compared with wt controls and no protein was detected in several tissues and hematopoietic cells from RIAM−/− mice including platelets, macrophages, and polymorphonuclear leukocytes (PMNs) (Figure 1A-D; supplemental Figure 1). Importantly, RIAM−/− platelets, PMNs, and macrophages expressed normal amounts of the integrin regulatory proteins talin-1 and kindlin-3, as well as Rap1 and its upstream regulator calcium and diacylglycerol (DAG)-regulated guanine nucleotide exchange factor 1 (CalDAG-GEFI) (Figure 1B-D; supplemental Figure 1). As recently reported,25 RIAM−/− mice were fertile, without an overt phenotype, showed a normal life span, and exhibited normal platelet counts, platelet aggregation, and αIIbβ3 integrin activation and binding to fibrinogen (data not shown) confirming that RIAM is dispensable for platelet integrin regulation. Interestingly, although RIAM is expressed at rather low levels in platelets and in many other tissues, western blotting showed high RIAM expression levels both in myeloid and lymphoid cells (Figure 1E).
RIAM is strongly expressed in cells of hematopoietic origin. (A-D) Western blot analysis confirms loss of RIAM protein in indicated tissues and cells of RIAM−/− mice. Levels of talin-1 (A-D), Cal-DAG (B), Kindlin-3 (B-D), and Rap1 (B-D) are unaffected in different organs (A), platelets (B), macrophages (C), and PMNs (D). RIAM levels are reduced in platelets, macrophages, and PMNs isolated from RIAM+/− mice (B-D). (E) Comparison of RIAM and talin-1 expression in different tissues and hematopoietic cells. GAPDH expression served as loading control. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; sk., skeletal.
RIAM is strongly expressed in cells of hematopoietic origin. (A-D) Western blot analysis confirms loss of RIAM protein in indicated tissues and cells of RIAM−/− mice. Levels of talin-1 (A-D), Cal-DAG (B), Kindlin-3 (B-D), and Rap1 (B-D) are unaffected in different organs (A), platelets (B), macrophages (C), and PMNs (D). RIAM levels are reduced in platelets, macrophages, and PMNs isolated from RIAM+/− mice (B-D). (E) Comparison of RIAM and talin-1 expression in different tissues and hematopoietic cells. GAPDH expression served as loading control. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; sk., skeletal.
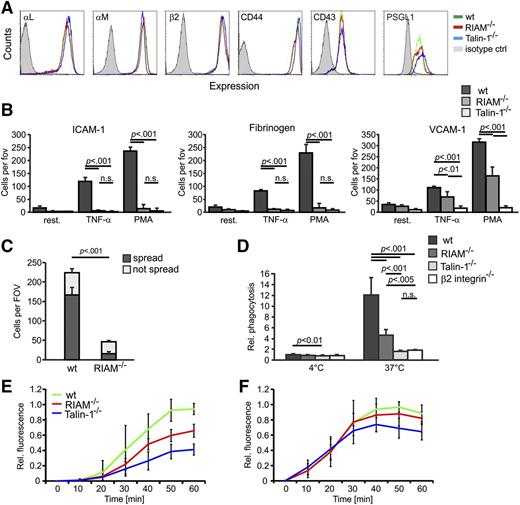
RIAM was shown to regulate integrins through controlling the activity of talin. Therefore, we measured peripheral blood (PB) cell counts and compared leukocyte adhesion and extravasation between wt and RIAM−/− mice and mice in which the floxed talin-1 gene was conditionally deleted in the hematopoietic system using the Mx1-Cre or in T cells using the CD4-Cre transgenic driver lines, respectively. The PB cell counts revealed a leukocytosis in RIAM−/− mice, which was more pronounced and accompanied by an anemia in poly-IC–induced talin-1fl/fl/Mx1-Cre mice (Table 1). Fluorescence-activated cell sorting (FACS) analyses revealed normal surface levels of the integrin subunits αL, αM, and β2 or other leukocyte adhesion molecules including CD44, CD43, and P-selectin glycoprotein ligand 1 (PSGL-1) on RIAM−/− PMNs (Figure 2A), and hence excluded a defect in integrin surface expression as cause for the leukocytosis. To determine whether loss of RIAM impairs leukocyte integrin functions leading to diminished leukocyte adhesion, we performed adhesion assays in the presence or absence of tumor necrosis factor-α (TNF-α) and phorbol-12-myristate-13-acetate (PMA). The experiments revealed that adhesion of RIAM−/− PMNs to the β2 integrin ligands intercellular cell adhesion molecule 1 (ICAM-1) and fibrinogen in response to TNF-α and PMA was abolished similarly as in talin-1−/− PMNs, whereas their adhesion to the α4β1 integrin ligand vascular cell adhesion molecule 1 (VCAM-1) was only partially impaired in RIAM−/− PMNs but completely abolished in talin-1−/− PMNs (Figure 2B). The failure of wt neutrophils to bind VCAM-1 in the presence of β1 integrin-blocking antibodies and β2 integrin-deficient PMNs to ICAM-1 or fibrinogen, respectively, confirms the well-known integrin specificity for ligand (supplemental Figure 2A-C). Interestingly, binding of the β1 integrin activation epitope reporting antibody 9EG7 to activated RIAM−/− PMNs was comparable to wt PMNs, whereas talin-1−/− PMNs showed no 9EG7 binding (see supplemental Figure 3A).
Integrin functions on RIAM−/− PMNs are severely impaired. (A) Representative FACS blots showing surface levels of αL integrin, αM integrin, β2 integrin, CD44, CD43, and PSGL-1 on wt (green), RIAM−/− (red), and talin-1−/− (blue) PMNs. BM cells were gated for high Gr1 expression. Isotype control staining is shown in light gray. (B) Static adhesion of untreated (resting), TNF-α–, and PMA-treated BM-derived PMNs from wt, RIAM−/−, and talin-1−/− mice on ICAM-1, fibrinogen, or VCAM-1 (n = 5 independent experiments). (C) Number of adherent (not spread) and spread wt and RIAM−/− PMNs seeded on immune complexes for 30 minutes were individually counted (n = 3 independent experiments). (D) Relative amount of fluorescently labeled Escherichia coli particles phagocytosed by wt, RIAM−/−, talin-1−/−, and β2 integrin−/− at 4°C and 37°C (n = 8, 8, 8, 3). (E-F) Oxidative burst measured in wt, RIAM−/−, and talin-1−/− PMNs after stimulation with TNF-α (E) and PMA (F), respectively. P values for TNF-α stimulation: wt vs RIAM−/− at 50 minutes (P < .005) and at 60 minutes (P < .001); wt vs talin-1−/− at 40 minutes (P < .005) and at 50 minutes and 60 minutes (P < .001), RIAM−/− vs talin-1−/− at 40 minutes and 50 minutes (P < .01) and at 60 minutes (P < .005). P values for PMA stimulation: wt vs talin-1−/− at 40/50/60 minutes (P < .05; n = 5). Data are shown as mean ± SD; P values indicate significant differences and were calculated with the Student t test. ctl, control; fov, field of view; n.s., not significant; Rel., relative; rest., resting.
Integrin functions on RIAM−/− PMNs are severely impaired. (A) Representative FACS blots showing surface levels of αL integrin, αM integrin, β2 integrin, CD44, CD43, and PSGL-1 on wt (green), RIAM−/− (red), and talin-1−/− (blue) PMNs. BM cells were gated for high Gr1 expression. Isotype control staining is shown in light gray. (B) Static adhesion of untreated (resting), TNF-α–, and PMA-treated BM-derived PMNs from wt, RIAM−/−, and talin-1−/− mice on ICAM-1, fibrinogen, or VCAM-1 (n = 5 independent experiments). (C) Number of adherent (not spread) and spread wt and RIAM−/− PMNs seeded on immune complexes for 30 minutes were individually counted (n = 3 independent experiments). (D) Relative amount of fluorescently labeled Escherichia coli particles phagocytosed by wt, RIAM−/−, talin-1−/−, and β2 integrin−/− at 4°C and 37°C (n = 8, 8, 8, 3). (E-F) Oxidative burst measured in wt, RIAM−/−, and talin-1−/− PMNs after stimulation with TNF-α (E) and PMA (F), respectively. P values for TNF-α stimulation: wt vs RIAM−/− at 50 minutes (P < .005) and at 60 minutes (P < .001); wt vs talin-1−/− at 40 minutes (P < .005) and at 50 minutes and 60 minutes (P < .001), RIAM−/− vs talin-1−/− at 40 minutes and 50 minutes (P < .01) and at 60 minutes (P < .005). P values for PMA stimulation: wt vs talin-1−/− at 40/50/60 minutes (P < .05; n = 5). Data are shown as mean ± SD; P values indicate significant differences and were calculated with the Student t test. ctl, control; fov, field of view; n.s., not significant; Rel., relative; rest., resting.
To confirm the crucial role of RIAM for β2 integrin-mediated adhesion, we allowed neutrophils to adhere and spread on immobilized immune complexes, which trigger Fcγ receptor-mediated inside-out activation of αMβ2 integrins followed by the binding to activated complement component iC3b. This assay mimics neutrophil adhesion to a complement-opsonized pathogen.29 Whereas wt neutrophils strongly adhered to and spread on the immune complexes, RIAM−/− neutrophils adhered and spread significantly less (Figure 2C), indicating that Fcγ receptor-mediated activation of αMβ2 integrins is significantly impaired. To further validate the essential role of RIAM for β2 integrin-mediated functions, we first measured phagocytosis of serum-opsonized bacteria and found that bacterial uptake by RIAM-deficient PMNs was strongly reduced although to a lesser extent than by talin-1−/− or β2 integrin−/− PMNs (Figure 2D). Similarly, treatment of wt PMNs with an anti-β2 integrin-blocking antibody reduced phagocytosis, although to a lesser extent than β2 integrin- or RIAM-deficient PMNs (supplemental Figure 2D). Second, we determined the β2 integrin-mediated degranulation and respiratory burst of free reactive oxygen species in response to TNF-α and found a significant reduction in RIAM−/− PMNs, however, again to a lesser extent than in talin-1−/− PMNs (Figure 2E). In contrast, PMA treatment, which induces a respiratory burst independent of β2 integrin signaling,30 induced a similar reactive oxygen species production in wt, RIAM−/−, and talin-1−/− PMNs, indicating that the molecular machinery to induce respiratory bursts is present and operating in these cells (Figure 2F). Altogether, these in vitro data indicate that RIAM is required for β2 integrin function in PMNs.
RIAM is required for leukocyte adhesion and extravasation in vivo
Next, we investigated whether RIAM−/− neutrophils also display defects in vivo. First, we connected glass capillaries coated with recombinant E-selectin in the absence or presence of ICAM-1 and the keratinocyte-derived chemokine (KC or CXC chemokine ligand [CXCL-1]) with the carotid artery of wt, RIAM−/−, and poly-IC–induced talin-1fl/fl/Mx1-Cre mice, respectively. This experimental setup evaluates chemokine-induced leukocyte activation, which results in integrin-mediated adhesion and arrest on glass-immobilized ligands under physiologic flow conditions.31 In the absence of the chemokine none of the leukocytes showed significant adhesion, however, in the presence of the immobilized chemokine, wt neutrophils strongly adhered to ICAM-1, whereas adhesion of RIAM−/− and talin-1−/− neutrophils to ICAM-1 was similarly abolished (Figure 3A). The adhesion of wt leukocytes to E-selectin–coated glass capillaries in the absence of ICAM-1 and KC is probably due to unspecific β2 integrin-mediated adhesion to the glass surface, as the presence of anti-β2 integrin-blocking antibodies abrogated adhesion (supplemental Figure 4).
RIAM−/− mice suffer from LAD and extravasation defects. (A) β2 integrin-mediated adhesion of PMNs from wt, RIAM−/−, and talin-1−/− mice under flow ex vivo. Flow chambers were coated with E-selectin only or a mixture of E-selectin, ICAM-1, and/or CXCL1 (K), connected to the carotid artery and perfused with whole blood for 10 minutes (n ≥ 4 chambers in 3 independent experiments for each condition). (B-C) Mean rolling velocity (B) and adhesion efficiency (C) of PMNs in TNF-α–stimulated postcapillary cremaster muscle venules of wt, RIAM−/−, and talin-1−/− mice (n = 4 independent experiments). (D) Quantification of perivascular PMNs after Giemsa staining of TNF-α–stimulated cremaster muscles 3 hours after cytokine application (n ≥ 5 independent experiments). (E) Adhesion efficiency of leukocytes in unstimulated cremaster muscle venules of wt and RIAM−/− mice before and 2 minutes after injection of the arrest chemokine CXCL1 (n = 4 of 6 independent experiments). (F) Adhesion efficiency of PMNs in TNF-α–stimulated postcapillary cremaster muscle venules of BM mixed chimeras analyzed 8 weeks after transplantation (n = 4 independent experiments). (G) Whole-mount staining of phorbol ester–treated ears from wt and RIAM−/− mice, stained with an anti-pan-laminin antibody (LN, green) to visualize endothelial basement membranes and an anti-Gr-1 antibody to identify neutrophils. Arrowheads indicate extravasated cells; arrows point to neutrophils within blood vessels. Scale bar represents 100 μm. (H-I) wt and RIAM−/− mice were exposed to aerosolized LPS for 30 minutes and ratios of neutrophils extravasated into the interstitium (H) or the brachioalveolar space (I) and neutrophils remaining in the lung vasculature are shown 4 hours after treatment. Data are shown as mean ± SEM P values indicate significant differences and were calculated with the Student t test. E, E-selectin; EI, E-selectin and ICAM-1; EIK, E-selectin, ICAM-1 and KC.
RIAM−/− mice suffer from LAD and extravasation defects. (A) β2 integrin-mediated adhesion of PMNs from wt, RIAM−/−, and talin-1−/− mice under flow ex vivo. Flow chambers were coated with E-selectin only or a mixture of E-selectin, ICAM-1, and/or CXCL1 (K), connected to the carotid artery and perfused with whole blood for 10 minutes (n ≥ 4 chambers in 3 independent experiments for each condition). (B-C) Mean rolling velocity (B) and adhesion efficiency (C) of PMNs in TNF-α–stimulated postcapillary cremaster muscle venules of wt, RIAM−/−, and talin-1−/− mice (n = 4 independent experiments). (D) Quantification of perivascular PMNs after Giemsa staining of TNF-α–stimulated cremaster muscles 3 hours after cytokine application (n ≥ 5 independent experiments). (E) Adhesion efficiency of leukocytes in unstimulated cremaster muscle venules of wt and RIAM−/− mice before and 2 minutes after injection of the arrest chemokine CXCL1 (n = 4 of 6 independent experiments). (F) Adhesion efficiency of PMNs in TNF-α–stimulated postcapillary cremaster muscle venules of BM mixed chimeras analyzed 8 weeks after transplantation (n = 4 independent experiments). (G) Whole-mount staining of phorbol ester–treated ears from wt and RIAM−/− mice, stained with an anti-pan-laminin antibody (LN, green) to visualize endothelial basement membranes and an anti-Gr-1 antibody to identify neutrophils. Arrowheads indicate extravasated cells; arrows point to neutrophils within blood vessels. Scale bar represents 100 μm. (H-I) wt and RIAM−/− mice were exposed to aerosolized LPS for 30 minutes and ratios of neutrophils extravasated into the interstitium (H) or the brachioalveolar space (I) and neutrophils remaining in the lung vasculature are shown 4 hours after treatment. Data are shown as mean ± SEM P values indicate significant differences and were calculated with the Student t test. E, E-selectin; EI, E-selectin and ICAM-1; EIK, E-selectin, ICAM-1 and KC.
Second, we tested whether loss of RIAM expression also affects the adhesion cascade and extravasation of neutrophils on TNF-α–stimulated cremaster muscle venules. The mean leukocyte rolling velocity, which is primarily determined by interactions of neutrophils with P- and E-selectins on endothelial cells, and to a lesser extent by α4β1 and αLβ2 integrin binding to VCAM-1 and ICAM-1,32 was significantly elevated for RIAM−/− PMNs, whereas adhesion efficiency (calculated as number of adherent neutrophils per mm2 vascular surface area divided by the systemic leukocyte numbers) and numbers of extravasated leukocytes in the perivascular cremaster muscle tissue were significantly decreased (Figure 3B-D). The severe adhesion defect of RIAM-deficient neutrophils was confirmed by injecting the neutrophil arrest-inducing chemokine KC into wt and RIAM−/− mice. Although KC further elevated the numbers of adherent neutrophils in postcapillary venules of the exteriorized cremaster muscle of wt mice, there was no change in RIAM−/− mice (Figure 3E). To exclude that the adhesion and extravasation defects of RIAM−/− neutrophils are due to loss of RIAM expression in endothelial cells, we generated BM chimeras by transplanting RIAM−/− BM cells into lethally irradiated wt recipient mice and vice versa (wt BM cells into lethally irradiated RIAM−/− mice). Although adhesion efficiencies of wt neutrophils behaved comparably in RIAM−/− and in wt recipient mice, the adhesion efficiency of RIAM−/− neutrophils was strongly impaired in wt recipient mice strongly indicating that the severe adhesion defect of RIAM−/− neutrophils is cell autonomous and due to an impaired activation of their β2 integrins (Figure 3F). Of note, rolling velocity and extravasation of neutrophils were significantly more compromised in talin-1−/− than in RIAM−/− neutrophils (Figure 3B-D), indicating that either additional talin activators are compensating the loss of RIAM and/or α4β1 integrin-mediated rolling is less affected in the absence of RIAM than in the absence of talin-1. Importantly, microvascular parameters were indistinguishable between groups (see supplemental Tables 1-3).
To further confirm the extravasation defect of RIAM−/− PMNs, we tested neutrophil extravasation in an acute skin inflammation model, which revealed that RIAM−/− PMNs remained within the vasculature of phorbol ester–treated ears, whereas wt PMNs readily left the vascular system and accumulated in the tissue (Figure 3G). Finally, we applied an acute lung injury model, in which wt and RIAM−/− mice were treated with aerosolized lipopolysaccharide (LPS) for 30 minutes to mimic a bacterial infection and then analyzed for leukocyte transmigration from the lung vasculature into the interstitium and the bronchoalveolar space. Also, these experiments demonstrated a severe extravasation defect of RIAM−/− PMNs (Figure 3H).
Altogether, these findings indicate that RIAM−/− mice suffer from leukocyte adhesion and extravasation deficiencies due to impaired β2 integrin-mediated adhesion to the vascular endothelium.
RIAM mediates static macrophage adhesion and spreading on ICAM-1
Monocyte/macrophages express considerable levels of β1, β2, and β3 integrins, which assemble special adhesion sites called podosomes on rigid substrates such as glass. Podosomes consist of a central actin core that is surrounded by a ring complex consisting of integrins and integrin-associated proteins such as talin-1, kindlin-3, and vinculin.33 To determine the subcellular localization of RIAM, we overexpressed enhanced green fluorescent protein (EGFP)-tagged RIAM in wt and RIAM-deficient BM-derived macrophages and dendritic cells (DCs) and found RIAM in the vinculin-containing podosomal ring structure (Figure 4A; supplemental Figure 5A). Although integrin activation is essential for podosome formation,34 a similar number of wt and RIAM-deficient DCs and macrophages assembled podosomes, which were organized in clusters of similar size (Figure 4A; supplemental Figure 5). RIAM overexpression also had no impact on podosome formation and organization (supplemental Figure 5F-G). In contrast, adhesion of RIAM−/− macrophages to ICAM-1 was strongly reduced and to VCAM-1 slightly reduced, whereas adhesion to fibronectin (FN) and vitronectin (VN) was unaffected (Figure 4B-E). The impaired adhesion of RIAM-deficient macrophages was normalized by bypassing inside-out integrin activation with Mn2+ on VCAM-1, whereas their adhesion on ICAM-1 was only partially rescued (Figure 4B-E). Of note, wt and RIAM−/− macrophages displayed comparable 9EG7 binding, which was strongly reduced in talin-1–deficient macrophages (see supplemental Figure 3B). To test macrophage spreading, we seeded RIAM-deficient macrophages on different ligand-coated surfaces and found normal spreading on FN, VN, and VCAM-1 and significantly diminished spreading on ICAM-1. Mn2+ treatment, however, rescued the spreading defect (Figure 4F-H), indicating that RIAM promotes primarily the activation of β2-class integrins in macrophages. In addition, we tested integrin-mediated outside-in signaling in BM-derived macrophages. To this end, starved wt and RIAM−/− macrophages were either kept in suspension or plated for 20 minutes on FN or ICAM-1 before they were lysed and adhesion triggered protein phosphorylation was analyzed by western blotting. Only minor differences could be observed in the global tyrosine phosphorylation profile in response to adhesion on both ligands (Figure 4I) and the absence of RIAM did neither affect FAK-Tyr397 nor Src-Tyr416 phosphorylation. In contrast, Pyk2 phosphorylation was strongly reduced upon adhesion and was only partially rescued when cell adhesion occurred in the presence of Mn2+ (Figure 4J-K), suggesting a role of RIAM also in integrin-mediated outside-in signaling. Furthermore, the significantly more pronounced adhesion defects of talin−/− macrophages and their less efficient rescue by Mn2+ in comparison with RIAM−/− macrophages (Figure 4B-F) indicates that additional talin activators must exist in macrophages that induce talin-mediated integrin functions.
Integrin functions are impaired on RIAM−/− macrophages. (A) Vinculin (red) and F-actin staining (phalloidin; blue) of wt and RIAM−/− BM-derived DCs expressing EGFP or RIAM-EGFP (green). Scale bar represents 5 μm. (B-E) Static adhesion assay of BM-derived wt, RIAM−/−, and talin-1−/− macrophages to FN (B), VN (C), VCAM-1 (D), and ICAM-1 (E) in the presence or absence of 0.5 mM MnCl2 relative to wt macrophages (n ≥ 5 independent experiments). Adhesion was measured indirectly using crystal violet staining and optical density (OD) measurement at 595 nm. (F) Representative images of macrophages seeded for 30 minutes on FN, VN, or VCAM-1 and on ICAM-1 in the absence or presence of 0.5 mM MnCl2. Scale bar represents 50 μm. (G) Mean ± SD values of spreading area of wt, RIAM−/−, and talin−/− macrophages seeded for 30 minutes on FN, VN, VCAM-1, or ICAM-1 (n = 5 independent experiments with 50 cells measured per experiment). (H) Mean ± SD values of spreading area of wt and RIAM−/− macrophages seeded for 30 minutes on ICAM-1 in the absence or presence of 0.5 mM MnCl2 (n = 3 independent experiments with 50 cells measured per experiment). (I-J) wt and RIAM−/− macrophages were either kept in suspension or plated for 20 minutes on FN or ICAM-1, and analyzed for global tyrosine phosphorylation (I) and for phosphorylation and total expression of Src, FAK, and Pyk2 (J). GAPDH served as loading control. (K) wt and RIAM−/− macrophages were either kept in suspension or plated for 20 minutes on FN or ICAM-1 in the presence of 0.5 mM MnCl2 and analyzed for Pyk2 phosphorylation. P values indicate significant differences and were calculated with the Student t test. sus., suspension.
Integrin functions are impaired on RIAM−/− macrophages. (A) Vinculin (red) and F-actin staining (phalloidin; blue) of wt and RIAM−/− BM-derived DCs expressing EGFP or RIAM-EGFP (green). Scale bar represents 5 μm. (B-E) Static adhesion assay of BM-derived wt, RIAM−/−, and talin-1−/− macrophages to FN (B), VN (C), VCAM-1 (D), and ICAM-1 (E) in the presence or absence of 0.5 mM MnCl2 relative to wt macrophages (n ≥ 5 independent experiments). Adhesion was measured indirectly using crystal violet staining and optical density (OD) measurement at 595 nm. (F) Representative images of macrophages seeded for 30 minutes on FN, VN, or VCAM-1 and on ICAM-1 in the absence or presence of 0.5 mM MnCl2. Scale bar represents 50 μm. (G) Mean ± SD values of spreading area of wt, RIAM−/−, and talin−/− macrophages seeded for 30 minutes on FN, VN, VCAM-1, or ICAM-1 (n = 5 independent experiments with 50 cells measured per experiment). (H) Mean ± SD values of spreading area of wt and RIAM−/− macrophages seeded for 30 minutes on ICAM-1 in the absence or presence of 0.5 mM MnCl2 (n = 3 independent experiments with 50 cells measured per experiment). (I-J) wt and RIAM−/− macrophages were either kept in suspension or plated for 20 minutes on FN or ICAM-1, and analyzed for global tyrosine phosphorylation (I) and for phosphorylation and total expression of Src, FAK, and Pyk2 (J). GAPDH served as loading control. (K) wt and RIAM−/− macrophages were either kept in suspension or plated for 20 minutes on FN or ICAM-1 in the presence of 0.5 mM MnCl2 and analyzed for Pyk2 phosphorylation. P values indicate significant differences and were calculated with the Student t test. sus., suspension.
RIAM is required for T-cell adhesion to ICAM-1 and for T-cell homing to LNs
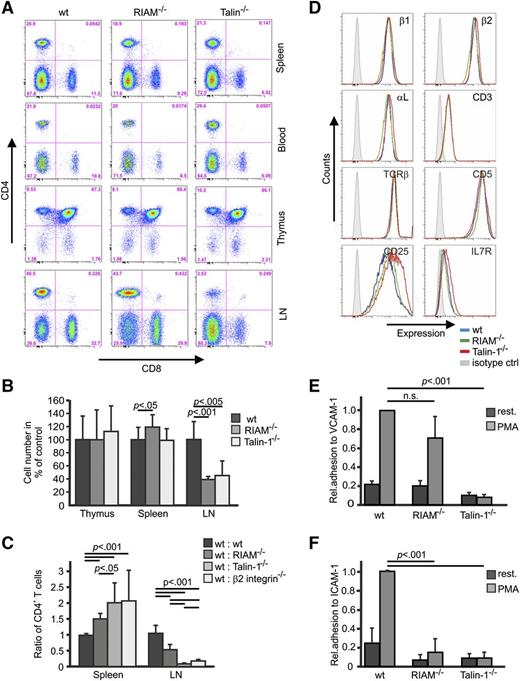
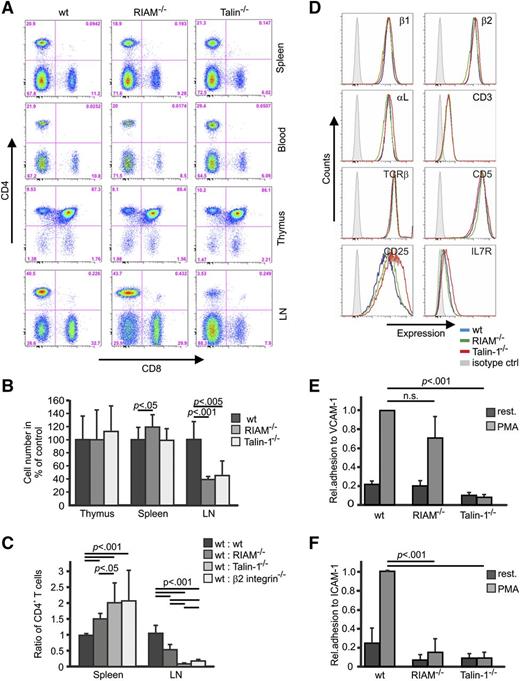
Lymphocytes were also elevated in the PB of RIAM-deficient mice. Furthermore, T-cell receptor (TCR) signaling was shown to induce integrin activation by promoting RIAM binding to Rap1 at the plasma membrane,17,35 pointing to an important role of the RIAM/talin complex for T cells. To test this possibility, we determined T-cell differentiation and their distribution in primary and secondary lymphatic organs and found that the percentage of CD4- and CD8-positive T cells in thymus, spleen, PB, and lymph nodes (LNs) was normal in RIAM-deficient mice (Figure 5A). Cellularity of the thymus was normal and slightly increased in spleen, whereas cell numbers in LNs of RIAM-deficient mice were significantly decreased (Figure 5B). To confirm that the reduced T-cell numbers in the LNs of RIAM−/− mice are due to impaired trafficking, we compared homing of adoptively transferred wt and RIAM-deficient T cells to LNs. In line with previous reports, which showed strongly reduced T-cell homing to LNs in the absence of talin-1 and β2 integrin, homing of RIAM-deficient CD4 T cells was significantly impaired and was mirrored by an accumulation of these T cells in the spleen (Figure 5C).36,37 Collectively, these observations strongly suggest that efficient entry of mature T cells into LNs is RIAM- and talin-1–dependent, whereas earlier differentiation of these T cells is RIAM- and talin-1 independent.36
RIAM−/− T cells display defective β2 and β1 integrin functions. (A) Distribution of CD4 and CD8 T cells in different tissues derived from wt, RIAM−/−, and talin-1fl/flCD4Cre+ mice. Life cells were gated and analyzed for CD4 and CD8 expression. (B) Cellularity of thymus, spleen, and LNs of the indicated genotypes, shown relative to wt (n ≥ 4 mice). (C) Cell Trace carboxyfluorescein diacetate succinimidyl ester (CFSE) and Far Red–stained splenocytes from wt or RIAM−/−, talin-1−/−, and β2 integrin−/−−/− mice were IV injected into wt recipients at a 1:1 ratio and the ratio of mutant to wt CD4-positive cells in spleen and LN was determined by FACS (n = 12, 9, 9, 6 mice). (D) Representative FACS blots showing surface levels of β1 integrin, β2 integrin, αL integrin, CD3, TCRβ, CD5, CD25, and IL7R on ConA-activated and CD4-positive wt (blue), RIAM−/− (green), and talin-1−/− (red) T cells. Isotype staining is shown in light gray. (E-F) Static adhesion of untreated and PMA-treated, ConA-stimulated, and CD4-positive wt, RIAM−/−, and talin-1−/− T cells on ICAM-1 and VCAM-1, respectively, relative to wt PMA-treated T cells (n = 3 independent experiments). Adherent cells were stained with crystal violet and the staining intensity was measured at OD of 595 nm. Data are shown as mean ± SD. P values indicate significant differences and were calculated with the Student t test.
RIAM−/− T cells display defective β2 and β1 integrin functions. (A) Distribution of CD4 and CD8 T cells in different tissues derived from wt, RIAM−/−, and talin-1fl/flCD4Cre+ mice. Life cells were gated and analyzed for CD4 and CD8 expression. (B) Cellularity of thymus, spleen, and LNs of the indicated genotypes, shown relative to wt (n ≥ 4 mice). (C) Cell Trace carboxyfluorescein diacetate succinimidyl ester (CFSE) and Far Red–stained splenocytes from wt or RIAM−/−, talin-1−/−, and β2 integrin−/−−/− mice were IV injected into wt recipients at a 1:1 ratio and the ratio of mutant to wt CD4-positive cells in spleen and LN was determined by FACS (n = 12, 9, 9, 6 mice). (D) Representative FACS blots showing surface levels of β1 integrin, β2 integrin, αL integrin, CD3, TCRβ, CD5, CD25, and IL7R on ConA-activated and CD4-positive wt (blue), RIAM−/− (green), and talin-1−/− (red) T cells. Isotype staining is shown in light gray. (E-F) Static adhesion of untreated and PMA-treated, ConA-stimulated, and CD4-positive wt, RIAM−/−, and talin-1−/− T cells on ICAM-1 and VCAM-1, respectively, relative to wt PMA-treated T cells (n = 3 independent experiments). Adherent cells were stained with crystal violet and the staining intensity was measured at OD of 595 nm. Data are shown as mean ± SD. P values indicate significant differences and were calculated with the Student t test.
In situ activated T cells (T blasts) interact with multiple β2 and β1 integrin ligands in the lymphoid tissue milieu. To test whether inside-out integrin-dependent adhesion of these CD4 T cell blasts is also impaired, we first activated CD4-positive splenic T cells from wt, RIAM−/−, and talin-1fl/fl/CD4 Cre+ mice with concanavalin A (ConA) and confirmed that expression levels of surface integrins and T-cell markers such as CD3, TCRβ, CD5, CD25, and interleukin 7 (IL7) receptor were similar to ConA-activated wt T cells (Figure 5D). Next, we activated integrins on ConA-treated T cells with PMA and seeded them on VCAM-1 and ICAM-1, respectively. The experiments revealed that the PMA treatment induced a comparable adhesion of ConA-activated wt and RIAM−/− T cells to VCAM-1, whereas adhesion of PMA-treated talin-1−/− T cells to VCAM-1 was abolished (Figure 5E). In sharp contrast, RIAM−/− and talin−/− T cells failed to adhere to ICAM-1 upon PMA treatment (Figure 5F), indicating that the RIAM/talin complex is essential for β2 integrin-mediated adhesion to ICAM-1, but is dispensable for α4β1 integrin-mediated adhesion to VCAM-1.
Discussion
Comprehensive biochemical, cell biological, and structural studies identified RIAM as an important component of the integrin inside-out signaling cascade directly upstream of talin, which in turn is believed to be essential for the activation of all integrin classes. Despite its proposed role in talin activation, loss of RIAM expression in mice neither affects their development nor leads to overt postnatal phenotypes including an expected bleeding tendency due to impaired platelet integrin activation.25 These observations suggest that the role of the RIAM/talin complex in integrin regulation is either more restricted than originally predicted from in vitro studies or that the absence of RIAM is efficiently compensated by other talin activators and thereby enables normal development and tissue homeostasis in the absence of RIAM.
To test whether the RIAM/talin complex contributes to integrin activation in either a cell type- or an integrin-specific manner, we generated RIAM-deficient mice and confirmed that their development, postnatal homeostasis, fertility, and lifespan are unaffected as has recently been described by Stritt et al.25 Despite the surprising finding that RIAM is dispensable for platelet integrin-mediated functions, genetic studies have demonstrated that Rap1-mediated activation of talin is essential for affinity modulation of αIIbβ3 on platelets as deletions of talin-1, Rap1B, or the guanine nucleotide exchange factor CalDAG-GEFI, which induces GTP loading and hence activation of Rap1, abrogate integrin activation on platelets and cause severe bleeding in mice.3,38-40 These findings strongly indicate that another as-yet-unidentified Rap1 effector protein links activated Rap1 with talin-mediated αIIbβ3 activation in platelets.
Although RIAM-deficient mice did not suffer from visible abnormalities, they exhibit significant leukocytosis (elevated numbers of circulating leukocytes) especially in their neutrophil populations. Peripheral white blood cell counts rise when leukocyte integrins are dysfunctional, resulting in leukocyte adhesion deficiency (LAD) and impaired leukocyte extravasation. LAD syndromes have been described in humans and mice and can be caused by loss-of-function mutations of β2 integrins, or in combination with bleeding by mutations of Rap1 pathways and loss of kindlin-3.6,26,41 Our study demonstrates that loss of talin-1 expression, which is indispensable for the activation of integrins expressed on platelets and leukocytes leads to severe LAD combined with severe bleeding,3,40 whereas loss of the Rap1 effector protein RIAM leads to a LAD without any measurable bleeding tendency. These findings raise the important conclusions that the RIAM/talin complex operates as an integrin-activating module both in a cell type- and an integrin-specific manner and that a still unknown talin-activating protein(s) trigger(s) the activity of αIIbβ3 on platelets.
Why is RIAM important for leukocytes and dispensable for platelets? We excluded that levels of Rap1 GEF, CalDAG-GEFI, or the RIAM family member lamellipodin are upregulated in platelets and compensate for the loss of RIAM (data not shown). Interestingly, RIAM levels are low in platelets whereas talin-1 is highly abundant, pointing to the presence of additional proteins that recruit talin to the platelet plasma membrane, which is assumed to be the key function of RIAM.19,21,24 Such functions could be fulfilled by members of the Grb7 family of adaptor proteins consisting of Grb7, Grb10, and Grb14. They share a similar modular structure with MRL proteins with a central pleckstrin homology, a putative Ras-association domain combined with an N-terminal proline-rich region.42 Although an interaction of Grb7 with talin or Rap1 has not been reported so far, the C-terminal Src homology 2 domain of Grb7 was shown to directly bind focal adhesion kinase (FAK), which in turn binds talin.43,44 In addition, local production of PIP2 by the lipid kinase PIP kinase type I γ (PIPKIγ) could recruit the talin head and repel the talin rod domain thereby relieving the talin autoinhibition.45 Finally, it is also possible that the high levels of vinculin may promote/maintain talin activation in the absence of RIAM,24 probably through binding to PIP2, which releases the autoinhibited state of vinculin, resulting in the binding to the talin rod domain.46 One has to critically note, however, that high levels of PIP2 and talin at the plasma membrane of activated platelets as well as vinculin-mediated activation of talin are probably not sufficient for the activation of αIIbβ3 integrin, given that CalDAG-GEFI and Rap1 are so essential for normal talin-dependent αIIbβ3 activation and platelet aggregation.38,41 Neither PIP2 production nor vinculin activation have been reported to be induced by CalDAG-GEFI and Rap1, suggesting that an unknown protein is involved in talin activation in platelets.
Our study clearly indicates an essential role of RIAM in β2 integrin regulation. Besides defective β2 integrin activation in the absence of RIAM, reduced phagocytosis and ROS production also suggest a role of RIAM in β2 integrin-mediated outside-in signaling. Previous studies revealed that phosphorylation of the protein tyrosine kinase Pyk2 depend on active β2 integrins.47-49 Consistently, RIAM-deficient macrophages exhibit defective Pyk2 phosphorylation in response to adhesion signaling, which could only partially be rescued by Mn2+ treatment of macrophages plated on ICAM-1. Interestingly, FAK and Src phosphorylation were normal in RIAM−/− macrophages, which implies that RIAM is part of a signaling complex specifically required for β2 integrin-mediated Pyk2 activation.
Our experiments with different leukocyte subsets demonstrate that β2-class integrins are more affected in talin-1−/− than in RIAM−/− PMNs, and that talin-1 deficiency abolishes the function of all integrin classes, explaining why talin-1−/− mice suffer from a more severe leukocytosis. Furthermore, they show that β2-class integrins are more affected than β1-class integrins in RIAM−/− PMNs. These findings are in line with previous studies showing that activation of α4β1 on T cells is not affected by CalDAG-GEFI silencing or Rap1 inhibition, whereas activation of αLβ2 is strongly impaired.50 The underlying reason for this integrin class-specific requirement of CalDAG-GEFI, Rap1, and RIAM is not clear. It is conceivable that different integrin classes localize to defined membrane compartments that are targeted by different signaling pathways and are therefore differently activated. One interesting membrane compartment, which operates independently of Rap1 and RIAM, could be the leukocyte microvilli. It has been shown that α4β1 integrins on T cells are enriched on microvilli during adhesion to VCAM-1 under flow conditions,51-53 whereas αLβ2 integrins are enriched in nonmicrovillar compartments. Future super-resolution studies in these leukocytes should reveal whether RIAM recruitment to the plasma membrane of these and other leukocytes takes place near αLβ2 rather than α4β1 assemblies such as leukocyte microvilli.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Susanne Bierschenk, Michal Grzejszczyk, and Yvonne Jansen for excellent technical assistance, Dr Roy Zent for carefully reading the manuscript, and the core facilities in the Koch Institute Swanson Biotechnology Center for support.
This work was supported by the Deutsche Forschungsgemeinschaft (Mo927/7-1, Sp621/4-1 and the SFB1123 TP A06/A08/A09), the Max Planck Society, National Institutes of Health (NIH) grant U54-CA112967, funds from the Ludwig Center for Molecular Oncology at Massachusetts Institute of Technology, and National Cancer Institute Core Grant P30-CA14051.
Authorship
Contribution: S.K. designed and performed most of the experiments, analyzed data, and contributed to the writing of the manuscript; M.P. designed and performed experiments and analyzed data; E.M.P. and F.B.G. generated the RIAM−/− mouse; M.S. designed and performed experiments, analyzed data, and contributed to the discussion; O.S. performed experiments; R.F. initiated the project, supervised the research, and wrote the paper; and M.M. designed and performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reinhard Fässler, Max-Planck-Institute of Biochemistry, Am Klopferspitz 18, D-82152 Martinsried, Germany; e-mail: faessler@biochem.mpg.de.