Key Points
The GR gene can be inactivated in Streptamer-selected CMV-specific CD8+ T cells using TALEN.
The GR gene inactivation endows T cells with resistance to the immunosuppressive effects of corticosteroids in vitro and in vivo.
Abstract
Cytomegalovirus (CMV) infection is responsible for substantial morbidity and mortality after allogeneic hematopoietic stem cell transplant. T-cell immunity is critical for control of CMV infection, and correction of the immune deficiency induced by transplant is now clinically achievable by the adoptive transfer of donor-derived CMV-specific T cells. It is notable, however, that most clinical studies of adoptive T- cell therapy exclude patients with graft-versus-host disease (GVHD) from receiving systemic corticosteroid therapy, which impairs cellular immunity. This group of patients remains the highest clinical risk group for recurrent and problematic infections. Here, we address this unmet clinical need by genetic disruption of the glucocorticoid receptor (GR) gene using electroporation of transcription activator–like effector nuclease (TALEN) messenger RNA. We demonstrate efficient inactivation of the GR gene without off-target activity in Streptamer-selected CMV-specific CD8+ T cells (HLA-A02/NLV peptide), conferring resistance to glucocorticoids. TALEN-modified CMV-specific T cells retained specific killing of target cells pulsed with the CMV peptide NLV in the presence of dexamethasone (DEX). Inactivation of the GR gene also conferred resistance to DEX in a xenogeneic GVHD model in sublethally irradiated NOD-scid IL2rγnull mice. This proof of concept provides the rationale for the development of clinical protocols for producing and administering high-purity genetically engineered virus-specific T cells that are resistant to the suppressive effects of corticosteroids.
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is a potentially curative treatment of high-risk hematologic malignancies, including leukemias, lymphomas, and myelodysplastic syndromes.1 A number of factors influence clinical outcomes, including history of exposure to cytomegalovirus (CMV). Human CMV is a β-herpesvirus with a seroprevalence of 30% to 70% in developed countries. CMV infection after HSCT can cause multiple complications, including gastroenteritis, pneumonitis, hepatitis, retinitis, and encephalitis.2,3 Pneumonitis is the most serious manifestation, with mortality rates of >80% without antiviral chemotherapy and >50% even with standard antiviral therapies.4
Patients at the highest risk of clinically problematic CMV infection include serologically positive recipients,5-7 recipients of T-cell-depleted grafts,8,9 and those receiving corticosteroids for the treatment of graft-versus-host disease (GVHD) post-HSCT.10 Thus, CMV infection occurs in 60% to 90% of CMV-seropositive patients, depending on whether T-cell depletion is performed or not. Despite modern preemptive treatment strategies, potentially life-threatening disease still occurs in 3% to 10% of HSCT recipients.11 Effective antiviral drug therapies are available, but their use is associated with renal toxicity, myelosuppression, and, less commonly, the emergence of drug resistance.12-15 There is, therefore, an important clinical need for therapies that speed the tempo of immune reconstitution to reestablish immunologic control of the virus.
Because T cell–mediated immunity has been shown to play a critical role in the sustained control of CMV infection,16-18 several groups have investigated strategies to generate CMV-specific T lymphocytes for adoptive immunotherapy. These studies demonstrated that CMV-specific T cells can be expanded or directly selected from seropositive donors, administrated safely, and can restore functional T-cell immunity when the primary problem is numerical deficiency.19-24 Of importance, virus-specific T cells engrafted, expanded, and conferred protective immunity despite therapeutic levels of calcineurin inhibitors (eg, cyclosporine, tacrolimus), suggesting that these agents are permissive to the desired therapeutic activity. Parallel advances have been made in situations in which the donor is CMV seronegative, either by expanding naïve CMV-reactive T cells or by the use of third-party donor cells.25,26 From a global perspective, however, most recurrent and refractory CMV infections occur in patients with significant GVHD,10 largely as a result of high-dose glucocorticoid therapy. Despite advances in our understanding of the pathophysiology of GVHD, glucocorticoids remain the most effective and favored initial therapy for the treatment of both acute and chronic GVHD.27-29 This methodology provides a major obstacle to adoptive antiviral immunotherapy because glucocorticoids suppress T cell numbers and function.
Glucocorticoids exert potent immunosuppressive effects after binding to the glucocorticoid receptor (GR), a ligand-activated transcription factor.30 Sequestered by a heat-shock protein (hsp) complex (hsp90/hsp70/FK506 binding protein 52), the GR translocates to the nucleus upon binding to glucocorticoids,31 where it exerts direct effects on gene expression (eg, the induction of annexin I and mitogen-activated protein kinase phosphatase 1) and indirect effects through interactions with other transcription factors (eg, nuclear factor κB and activator protein 1). Other evidence supports nongenomic mechanisms of action involving second-messenger cascades (eg, the phosphatidylinositol 3-kinase/protein kinase B/endothelial nitric oxide synthase pathway).32-34 It is notable that the GR is essential for the immunosuppressive effects of glucocorticoids in T cells and that loss of GR function prevents all of their apoptotic actions.34,35 Despite the wide range of gene targets for the GR, only a single GR gene has been described, which generates several GR isoforms via alternative splicing.36 On the basis of these considerations, we hypothesized that selective inactivation of the GR gene in CMV-specific T cells would render them resistant to the suppressive effects of corticosteroids, thus providing a therapeutic option for the treatment of CMV infection in the context of glucocorticoid administration.
Powerful tools for genome editing are now available, including the transcription activator–like effector nucleases (TALENs).37,38 TALENs are attractive reagents for therapeutic applications because of their high specificity conferred by the long targeting domains (17-bp target sequence) and the requirement for homodimerization of the associated FokI domain–containing nucleases. The endonucleases cause site-specific double-stranded DNA breaks and trigger natural DNA repair mechanisms, notably nonhomologous end joining recombination that is error prone. The introduction of insertions or deletions (indels) can then shift the reading frame and inactivate the gene function.39 The success rate and activity of TALENs depend heavily on the cell type and delivery method.40 Transfection by electroporation with messenger RNA (mRNA) is an attractive method for therapeutic application because of its reproducibility and cost effectiveness.
We developed a highly efficient TALEN pair specific for the exon 2 sequence of the human GR gene. We demonstrated that after mRNA transfection, GR-targeting TALENs induce disruption of the GR gene at high frequencies of 55% and 60% in primary human T cells and in the human HLA-A02 T-cell lymphoma (T2) cell line, respectively. After dexamethasone (DEX) selection, the enriched subset of CMV-specific GR exon 2–targeted (GR-Ex2) T cells were resistant to glucocorticoids while maintaining their HLA-A02–restricted cytotoxic activity toward CMV peptide-pulsed (pp65) targets. Lastly, the in vivo resistance and functionality of the TALEN-modified CD8+ T cells were confirmed in a preclinical murine model of xenogeneic GVHD (xeno-GVHD). These data demonstrate the feasibility of generating CMV-specific T cells resistant to glucocorticoids within 10 days and further show that their functional activity remains intact.
Methods
Cell culture and transfection
The T2 cell line was provided by Dr Martin Pule and maintained in RPMI 1640 supplemented with 10% fetal calf serum (Gibco), l-glutamine, and penicillin-streptomycin (Sigma). CD8+ T cells were selected by positive selection using human CD8 MicroBeads (Miltenyi Biotec) according to the manufacturer. The cells were stimulated with Dynabeads Human T-Activator CD3/CD28 (Invitrogen) at 37°C in 5% carbon dioxide at 1 × 106 cells per milliliter for 4 days. Cells were kept at 1 × 106 cells per milliliter in complete RPMI 1640 supplemented with recombinant human interleukin-2 (rhIL-2). HLA-A02/NLV–specific T-cell clones were selected from healthy donors; peripheral blood mononuclear cells were separated by gradient centrifugation using Ficoll-Paque Plus (GE Healthcare), and HLA-A02/NLV–specific T-cell clones were selected with Strep-Tactin Magnetic Nanobeads for major histocompatibility complex class I Streptamer HLA-A02/NLV: NLVPMVATV (NLV; 6-7001-015; IBA Life Sciences) according to manufacturer instructions. Clonal populations were then expanded in vitro using the rapid expansion protocol.41 A total of 5 × 104 CD8+ T cells were added to cloning mix consisting of 5 × 106 irradiated CD19+ Epstein-Barr virus–transformed lymphoblastoid cell line (TM-LCL) cells, 25 × 106 irradiated allogeneic peripheral blood mononuclear cells, 30 ng/mL of anti-human CD3 (OKT3; Bio X Cell), and 20 ng/mL of rhIL-2. TM-LCL cells were generated using concentrated B95-8 virus supernatant and complete RPMI 1640 containing 1 μg/mL of cyclosporin A (Sigma). One week after rapid expansion protocol, 5 × 106 HLA-A02/NLV–specific T cells were resuspended in 200 μL of Cytoporation Medium T and electroporated with 200 μg of green fluorescent protein (GFP) mRNA (mock transfection) or with 10 μg of each TALEN mRNA in 0.4-cm gap MicroPulser cuvettes (Bio-Rad Laboratories) using a BTX AgilePulse electroporator (Harvard Apparatus) delivering several square wave pulses. The cells were then transferred into complete RPMI 1640 medium with 20 ng/mL of rhIL-2 and 1% human serum. On day 2, the cells were resuspended, as previously described, to remove the Cytoporation Medium T. On day 3, the cells were washed42,43 and the GR gene–inactivated T cells were enriched by 5 days of treatment with 10−4 M DEX (Sigma) in complete RPMI 1640 medium.
CFSE-based cytotoxicity assay
A total of 5 × 104, 5 × 103, or 5 × 102 HLA-A02/NLV-specific effector T cells or CMVGR-Ex2 T cells were added to a 96-well round-bottom plate (Thermo Scientific) and incubated or not with 10−4 M DEX for 72 hours in complete RPMI 1640. T2GR-Ex2 cells, serving as target antigen-presenting cells, were pulsed with 1 μg/mL of pp65 peptide or not in a cell culture medium for 4 hours at 37°C. T2GR-Ex2 pp65-pulsed (specific target) cells were labeled with 1 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes), and the T2GR-Ex2 nonpulsed (unspecific target) cells were labeled with 10 μM CFSE for 10 minutes at 37°C.44 CFSE-labeled target cells were resuspended at 5 × 103/100 μL and cocultured with the effector cells for 16 hours. A quantitative analysis of the cell populations was assessed by adding a fixed amount (1 × 104) of Cell Sorting Set-up Beads. The percentage of specific lysis was calculated as follows: % specific lysis = 1 − [(absolute number of CFSE-low specific-target cells)/(absolute number of CFSE-high unspecific-target cells)] × 100.
In vivo resistance and xeno-GVHD in NSG mice
A total of 5 × 106 T2 wild-type (T2wt) cells or 5 × 106 T2GR-Ex2 cells were IV injected into sublethally irradiated (2 Gy) NOD-scid IL2rγnull (NSG) mice, treated daily with 15 mg/kg of DEX intraperitoneally injected or not for 12 days. A total of 1 × 106 CD8+wt T cells or CD8+GR-Ex2 T cells were IV injected into irradiated NSG mice. Mice received DEX treatment as previously described for 21 days. Spleens were harvested and weighed, and the absolute number of human CD8+ T was quantified using beads. The human cells were selected from spleens using human CD2 MicroBeads and MS columns (MiltenyiBiotec) according to manufacturer instructions. In all experiments, mice were monitored for the appearance of xeno-GVHD–like symptoms, including hair loss, hunched posture, and reduced mobility. For additional information, see supplemental Material and Methods, available on the Blood Web site.
Results
Efficient inactivation of the GR in T2 cells after mRNA-mediated TALEN delivery
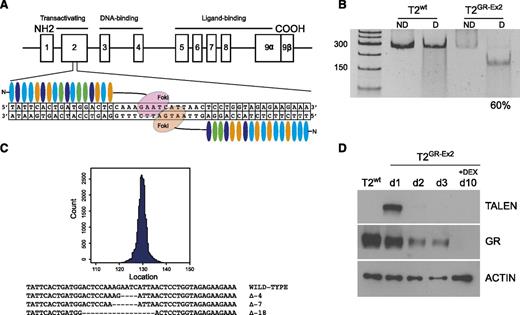
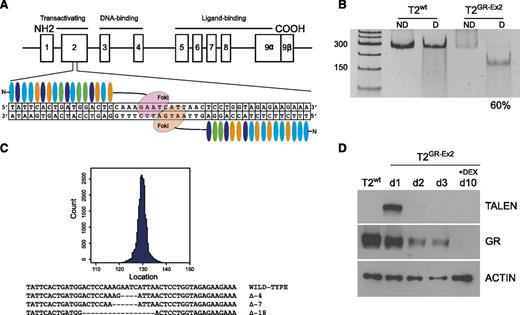
To inactivate the GR gene, 6 pairs of TALENs were designed to target different genomic sequences corresponding to various domains of the GR protein (transactivation domain, DNA binding domain, and ligand binding domain). Each pair was transfected into HEK-293 cells (2.5 μg of each half-TALEN) to allow high-throughput sequencing analysis. Those targeting the exon 2 sequence caused up to 28.2% targeted mutagenesis in the GR gene (see supplemental Table 1), without apparent cell toxicity and were selected for subsequent evaluation (Figure 1A). TALENs were then delivered into the T2 cell line using an optimized mRNA-electroporation protocol. Indel content analysis using the T7 endonuclease I assay revealed high TALEN efficacy, with mutation of up to 60% of the GR genomic sequence by 3 days after the transfection and in the absence of a selection marker (Figure 1B). Targeted deep-sequencing analysis (MiSeq) confirmed the high specificity of the TALENs for on-target mutagenesis, with dominant presence of 4- to 18-bp deletions (Figure 1C). Western blot analysis demonstrated a transient expression of the TALEN protein 1 and 2 days after transfection and a 50% decrease of the GR protein expression 72 hours after transfection. Lastly, an in vitro selection in high-dose (10−4 M) DEX for 7 days starting 72 hours after transfection significantly enriched the population of T2GR-Ex2 cells (Figure 1D; supplemental Figure 1A). It should be noted that culturing T2wt cells (GFP mRNA mock transfected) in DEX resulted in too few viable cells for subsequent experiments (supplemental Figure 1B).
Efficient TALEN-mediated editing of GR gene in the T2 cell line. (A) Schematic representation of the GR genomic locus (chromosome 5q31-q32) and TALEN-targeted sequence (exon 2). (B) T7 endonuclease I assay showing efficient nonhomologous end joining recombination–mediated mutagenesis (60%) at the intended target site on the GR gene. mRNA (10 μg) from each TALEN was used to transfect the T2 cell line with a BTX AgilePulse electroporator. At day 3, the genomic DNA was amplified by polymerase chain reaction and subjected to a mismatch-sensitive T7 endonuclease I digestion, prior to a separation on a 10% polyacrylamide Tris-borate-EDTA gel. Gene modification (indels) quantification was based on relative band intensities (ImageJ). (C) Representative sequences of the human GR on targeted site using MiSeq analysis. (D) Western blot analysis of TALEN and GR expression before and after DEX enrichment of mutated cells. At day 3, the T2GR-Ex2 (TALEN-modified) cells were treated with DEX (10−4 M) for 7 days. COOH, carboxylic acid; D, digested; ND, nondigested; NH2, amidogen.
Efficient TALEN-mediated editing of GR gene in the T2 cell line. (A) Schematic representation of the GR genomic locus (chromosome 5q31-q32) and TALEN-targeted sequence (exon 2). (B) T7 endonuclease I assay showing efficient nonhomologous end joining recombination–mediated mutagenesis (60%) at the intended target site on the GR gene. mRNA (10 μg) from each TALEN was used to transfect the T2 cell line with a BTX AgilePulse electroporator. At day 3, the genomic DNA was amplified by polymerase chain reaction and subjected to a mismatch-sensitive T7 endonuclease I digestion, prior to a separation on a 10% polyacrylamide Tris-borate-EDTA gel. Gene modification (indels) quantification was based on relative band intensities (ImageJ). (C) Representative sequences of the human GR on targeted site using MiSeq analysis. (D) Western blot analysis of TALEN and GR expression before and after DEX enrichment of mutated cells. At day 3, the T2GR-Ex2 (TALEN-modified) cells were treated with DEX (10−4 M) for 7 days. COOH, carboxylic acid; D, digested; ND, nondigested; NH2, amidogen.
TALEN-modified T2 cells are resistant to glucocorticoids
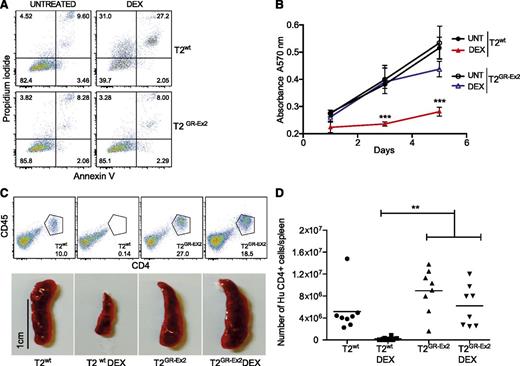
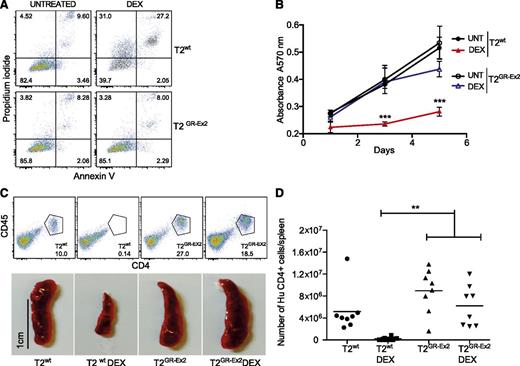
The T2GR-Ex2–enriched population of cells and control T2wt cells were incubated for 72 hours in vitro in the presence or absence of DEX (10−4 M). Although 50% of the T2wt cells were sensitive to DEX-induced apoptosis and secondary necrosis, the TALEN-modified T2GR-Ex2 cells were DEX resistant (Figure 2A; supplemental Figure 1B). A dose-response cell death assay with DEX or prednisone (10−5 M, 10−4 M, 10−3 M) confirmed that the T2GR-Ex2 cells were specifically resistant to both glucocorticoids (supplemental Figure 1B). Furthermore, the ability of T2wt cells to proliferate in vitro, highlighted by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide metabolic assay, was significantly reduced in the presence of DEX, whereas T2GR-Ex2 cells were DEX resistant, proliferating at levels equivalent to untreated T2wt cells (Figure 2B; supplemental Figure 1C). To assess whether T2GR-Ex2 cells were also resistant to glucocorticoids in vivo, T2wt and T2GR-Ex2 cells were IV injected into immunodeficient NSG mice that were either left untreated or treated with DEX for 12 days. After T-cell infusion and in the absence of DEX, spleens were heavily infiltrated by human CD4+/CD45+ T2wt cells or T2GR-Ex2 cells. In contrast, in mice receiving DEX, splenic infiltration was significantly reduced in the T2wt group but remained unaffected in the group of mice injected with T2GR-Ex2 cells (Figure 2C), supporting the in vitro data demonstrating resistance to glucocorticoid-induced cell death. Lastly, quantification of the absolute number of live human CD4+ cells in the spleens confirmed the macroscopic findings, further demonstrating the resistance of T2GR-Ex2 cells to DEX in vivo in comparison to T2wt cells (Figure 2D). These results show a potential proof of concept for the rapid production of glucocorticoid-resistant T cells, using an optimized mRNA-electroporation protocol and TALENs.
TALEN-modified T2-cell resistance to DEX in vitro and in vivo. (A) Apoptosis of T2wt cells and T2GR-Ex2 cells measured by annexin V and propidium iodide staining. Cells were cultured with DEX (10−4 M) for 72 hours, and the proportion of cell death was determined as positivity (right quadrants) for propidium iodide, annexin V, or both. (B) Time-response curves for cell proliferation of T2wt (closed symbols) or T2GR-Ex2 (open symbols) treated with DEX by using a typical 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are represented as means ± standard deviation from 3 independent experiments; statistical analysis was performed by 1-way ANOVA (***P < .001). (C) Splenic engraftment of T2wt or T2GR-Ex2 cells stained by human anti-CD45 and anti-CD4. Photographs depict the smaller size of harvested spleens from DEX-treated T2-injected recipients. A total of 5 × 106 T2wt or T2GR-Ex2 cells were IV injected into irradiated NSG mice treated daily with intraperitoneally injected DEX at 15 mg/kg or vehicle for 12 days. (D) Total number of human CD4+ cells per spleen. Data depict means from 2 independent experiments with 4 mice per group; statistical analysis was performed by 1-way ANOVA (**P < .01) with Bonferroni multiple comparisons (a nonparametric Student t test between T2 and T2 DEX groups revealed a P value of .0034). ANOVA, analysis of variance.
TALEN-modified T2-cell resistance to DEX in vitro and in vivo. (A) Apoptosis of T2wt cells and T2GR-Ex2 cells measured by annexin V and propidium iodide staining. Cells were cultured with DEX (10−4 M) for 72 hours, and the proportion of cell death was determined as positivity (right quadrants) for propidium iodide, annexin V, or both. (B) Time-response curves for cell proliferation of T2wt (closed symbols) or T2GR-Ex2 (open symbols) treated with DEX by using a typical 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are represented as means ± standard deviation from 3 independent experiments; statistical analysis was performed by 1-way ANOVA (***P < .001). (C) Splenic engraftment of T2wt or T2GR-Ex2 cells stained by human anti-CD45 and anti-CD4. Photographs depict the smaller size of harvested spleens from DEX-treated T2-injected recipients. A total of 5 × 106 T2wt or T2GR-Ex2 cells were IV injected into irradiated NSG mice treated daily with intraperitoneally injected DEX at 15 mg/kg or vehicle for 12 days. (D) Total number of human CD4+ cells per spleen. Data depict means from 2 independent experiments with 4 mice per group; statistical analysis was performed by 1-way ANOVA (**P < .01) with Bonferroni multiple comparisons (a nonparametric Student t test between T2 and T2 DEX groups revealed a P value of .0034). ANOVA, analysis of variance.
TALEN-mediated GR inactivation in primary CMV-specific T cells
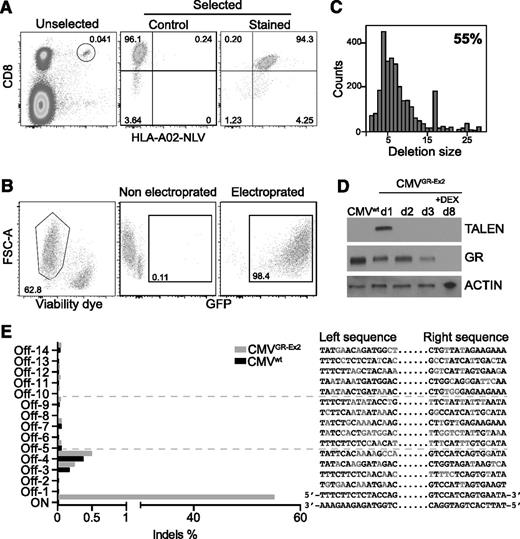
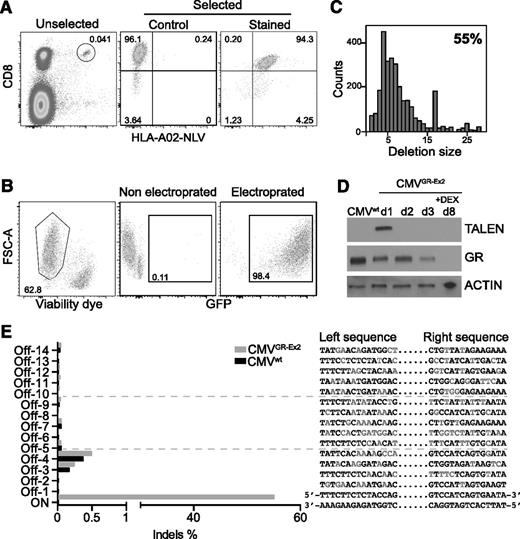
Clinical application of CMV-specific T-cell therapies after HSCT requires selection or selective expansion of virus-specific T cells in order to limit the number of potentially alloreactive T cells that are transferred. The necessity to avoid infusing alloreactive T cells is even more pertinent in patients who already have GVHD. Selection of CMV-specific T cells based on T-cell receptor specificity using HLA multimers (Streptamers) delivers a high-purity product, potentially mitigating risk in this setting. This technology depends on reversible binding of HLA multimer–peptide complexes coupled to magnetic selection, isolating peptide-specific T cells that are phenotypically and functionally indistinguishable from untreated cells. We therefore selected primary CD8+ CMV-specific T cells from healthy donors using HLA Streptamers directed against an immunodominant HLA-A02-restricted epitope derived from human CMV protein pp65 (NLV) (Figure 3A).
TALEN-mediated GR editing in Streptamer-selected CMV-specific CD8+ T cells. (A) Major histocompatibility complex class I Streptamers (HLA-A02) allow isolation of CMV-specific (NLV peptide) CD8+ T cells in high purity from donor blood leukocytes. (B) Primary T-cell transfection efficacy (>98%) and viability (>60%), using electroporation with in vitro transcribed mRNA. (C) Representative TALEN-induced nonhomologous end joining recombination mutagenesis in Streptamer-selected primary T cells by MiSeq analysis. (D) Western blot analysis of TALEN and GR expression before and after DEX enrichment of mutated cells. (E) Off-target site analysis from in silico prediction after next-generation sequencing. FSC-A, forward scatter area.
TALEN-mediated GR editing in Streptamer-selected CMV-specific CD8+ T cells. (A) Major histocompatibility complex class I Streptamers (HLA-A02) allow isolation of CMV-specific (NLV peptide) CD8+ T cells in high purity from donor blood leukocytes. (B) Primary T-cell transfection efficacy (>98%) and viability (>60%), using electroporation with in vitro transcribed mRNA. (C) Representative TALEN-induced nonhomologous end joining recombination mutagenesis in Streptamer-selected primary T cells by MiSeq analysis. (D) Western blot analysis of TALEN and GR expression before and after DEX enrichment of mutated cells. (E) Off-target site analysis from in silico prediction after next-generation sequencing. FSC-A, forward scatter area.
pp65-Specific CD8+ T cells were subsequently expanded in vitro for 1 week and then electroporated with 20 μg of GFP mRNA or 10 μg of each half-TALEN mRNA. The optimized protocol provided both high viability (median, > 60%) and a very high transfection efficacy (median, 98%), overcoming the frequently reported limitations (toxicity and low transfection efficacy) of primary T-cell engineering (Figure 3B). MiSeq analysis of the targeted exon 2 sequence showed 55% nonhomologous end joining recombination mutagenesis 3 days after transfection with the mRNA TALENs and characterized the different deletions induced at the specific on-target site (35 insertions were also reported in the 6046 reads; data not shown) (Figure 3C; supplemental Figure 2A). Analysis of the mutational rate by T7 endonuclease I digestion before and after selection with DEX highlighted an increase in the percentage of mutated CMVGR-Ex2 cells, from 60% to 89%, confirming the enrichment of GR gene–inactivated T cells under DEX selection pressure (supplemental Figure 2B). Mirroring the data in the T2GR-Ex2 cells, protein analysis assessed by western blot analysis demonstrated a transient expression of the TALEN proteins in the CMVGR-Ex2 cells. Similarly, 72 hours after transfection, we observed a 50% decrease of GR protein expression, and after 5 days of culture with DEX, no GR protein was detectable in the CMVGR-Ex2 cells by western blot analysis (Figure 3D; supplemental Figure 2C). In addition, the potential off-target sites of the GR-Ex2 TALEN in the human genome were characterized in silico, and the 14 most likely off-target cleavage sites, conserved in the seed region and containing 7 to 8 base mismatches, were ranked (supplemental Table 3). We measured the frequencies of small indels at the on-target site and putative off-target sites using deep sequencing. Indel event frequencies at the 14 potential off-target sites in the TALEN-treated cells (CMVGR-Ex2 cells) were identical to the ones observed in the mock-transfected cells (CMVwt cells), demonstrating the specificity of cleavage of the GR-Ex2 TALEN (Figure 3E; supplemental Tables 3 and 4).
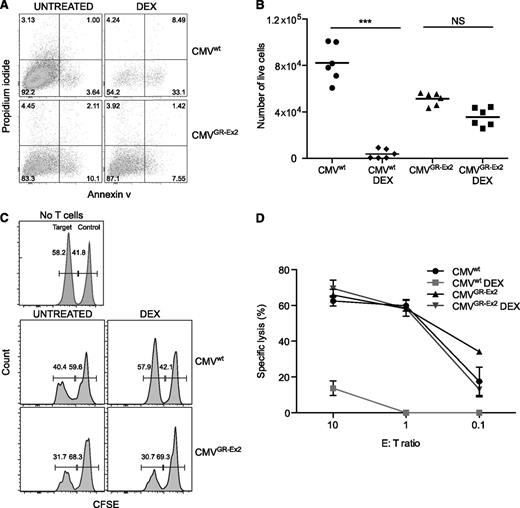
TALEN-modified CMVGR-Ex2 cells are resistant to DEX-induced immunosuppressive effects and retain cytolytic activity
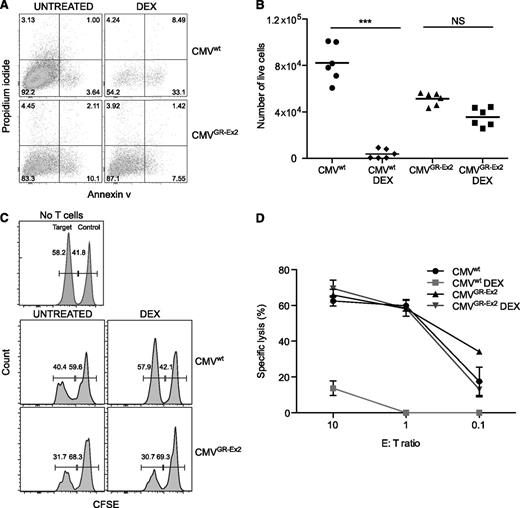
The resistance of CMVGR-Ex2 cells to DEX was quantified in an apoptosis assay. After 72 hours of exposure to DEX, no significant difference was observed in terms of annexin V labeling or absolute number of live cells between the CMVGR-Ex2 cells treated with or without DEX, in contrast to the impact of DEX on CMVwt cells (Figure 4A-B; supplemental Figure 2D). We also analyzed both granzyme B (GZB) expression and interferon-γ production in vitro. CMVwt and CMVGR-Ex2 cells were treated with DEX for 30 hours (before too few viable cells remain for subsequent analysis), prior to overnight stimulation with TM-LCL cells pulsed with the NLV peptide. Although both GZB and cytokine expression were significantly reduced in CMVwt cells by the addition of DEX, GZB and cytokine expression (supplemental Figure 2E,G-I) and the absolute number of cells (supplemental Figure 2F) were not significantly affected in CMVGR-Ex2 by the addition of DEX. To test the functional activity of the CMVGR-Ex2 cells in the presence or absence of DEX, we used the glucocorticoid-resistant T2GR-Ex2 cells generated in earlier experiments to assess target-specific cytolysis. T2 cells are deficient in the transporter associated with antigen transport protein but still express low amounts of major histocompatibility complex class I (HLA-A02) on their surface, which can be stabilized after loading with exogenous cognate peptides. Target T2GR-Ex2 cells were pulsed or not with the HLA-A02-restricted pp65 NLV peptide, labeled with 2 different concentrations of CFSE (pp65 pulsed, CFSE-low; unpulsed, CFSE-high) and mixed in a 1:1 ratio. The mixed population was incubated with an increasing number of CMVwt or CMVGR-Ex2 cells treated or not with DEX before and during the coculture. The NLV-specific lytic activity of the CMVGR-Ex2 cells was comparable to that of the CMVwt cells, but it was not affected by the DEX treatment (in contrast to CMVwt cells). High specific killing activity (60%) was maintained, even at a 1:1 effector/target ratio (Figure 4C-D).
Resistance to DEX and cytolytic activity of TALEN-modified CMV-specific CD8+ T cells. (A) Apoptosis of CMV-specific T cells (CMVwt) and CMV-specific TALEN-modified T cells (CMVGR-Ex2) measured by annexin V and propidium iodide staining after 72 hours in the presence of DEX (10−4 M). (B) Absolute number of live cells after 72 hours of DEX treatment measured by anti-CD8 staining and cell-counting beads. (C) Antigen-specific killing assay using CFSE-labeled target cells. The T2GR-Ex2 cell line was pulsed with the pp65 (NLV) viral peptide for 1 hour and CFSE labeled prior to coculture with CMV-specific T cells under DEX for 72 hours. (D) Dose response of specific cytolytic activity under DEX. Data are represented as means ± standard deviation from 3 independent experiments; statistical analysis was performed by 1-way ANOVA (***P < .001). E:T, effector/target; NS, not significant.
Resistance to DEX and cytolytic activity of TALEN-modified CMV-specific CD8+ T cells. (A) Apoptosis of CMV-specific T cells (CMVwt) and CMV-specific TALEN-modified T cells (CMVGR-Ex2) measured by annexin V and propidium iodide staining after 72 hours in the presence of DEX (10−4 M). (B) Absolute number of live cells after 72 hours of DEX treatment measured by anti-CD8 staining and cell-counting beads. (C) Antigen-specific killing assay using CFSE-labeled target cells. The T2GR-Ex2 cell line was pulsed with the pp65 (NLV) viral peptide for 1 hour and CFSE labeled prior to coculture with CMV-specific T cells under DEX for 72 hours. (D) Dose response of specific cytolytic activity under DEX. Data are represented as means ± standard deviation from 3 independent experiments; statistical analysis was performed by 1-way ANOVA (***P < .001). E:T, effector/target; NS, not significant.
TALEN-modified CD8+GR-Ex2 T cells are resistant to DEX in a xeno-GVHD model
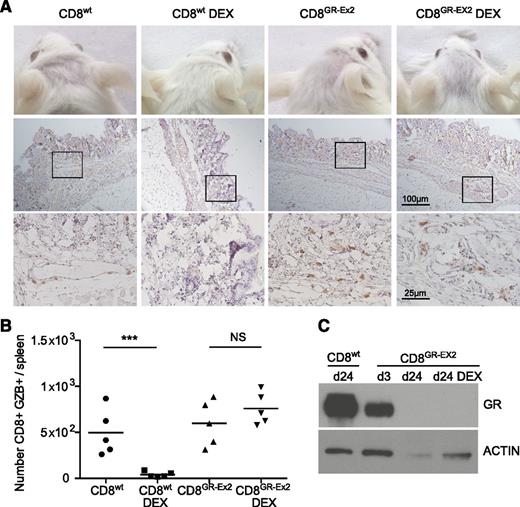
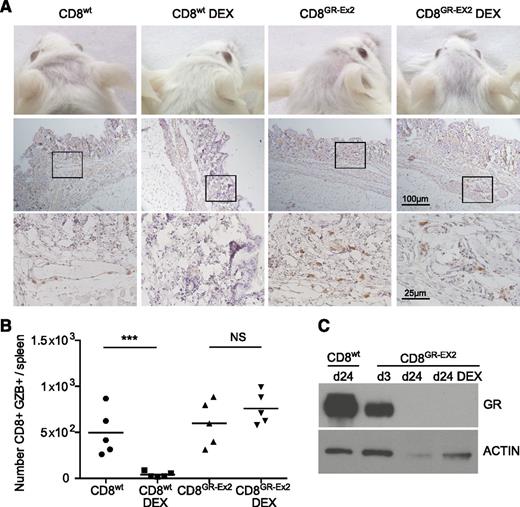
To measure glucocorticoid resistance and functional activity of polyclonal human CD8+T cells in vivo, 1 × 106 CD8+wt or CD8+GR-Ex2 T cells, generated as previously described, were IV injected into irradiated NSG mice treated or not with DEX for 21 days. These experiments also address a potential safety concern regarding the infusion of glucocorticoid-resistant alloreactive T cells. In human-mouse xeno-GVHD, human immune cell infiltration has been reported in peripheral tissues such as the skin,45-47 mimicking the human form of the disease.48 In human acute GVHD, the cutaneous lesions often appear 2 to 3 weeks post-HSCT, which is recapitulated in the xeno-GVHD model in NSG mice, where increasing infiltration by human cells is observed from day 14 to 28.42 Engraftment is associated with the development of acute xeno-GVHD syndrome, characterized by rapid weight loss (>10%), hunched posture, reduced mobility, and signs of cutaneous GVHD pathology such as hair loss.45 We therefore evaluated development of xeno-GVHD by the occurrence of hair loss, CD45+ (human) cell infiltration in the skin, spleen index, splenic engraftment of human CD8+ cells, and GZB expression. As expected, treatment with DEX for 21 days ablated engraftment and function of CD8+wt T cells, consistent with glucocorticoid sensitivity. Hair loss, skin infiltration with human CD45+ cells (score, 0.5-1.5), and a low but consistent level of splenic engraftment with the CD8+ population (range, 2%-4%) were observed in all mice receiving CD8+wt T cells or CD8+GR-Ex2 T cells in the absence of DEX, and in mice receiving CD8+GR-Ex2 T cells treated with DEX (Figure 5A; supplemental Figure 3). Extensive fibrosis, loss of normal splenic architecture, and the presence of a GZB+ CD8 population were also documented in these 3 cohorts (Figure 5B; supplemental Figure 3D). Human CD8+GR-Ex2 and CD8+wt T cells were selected from pooled spleens using human anti-CD2 beads, analyzed for GR expression by western blot analysis and compared with CD8+GR-Ex2 at day 3 after transfection with TALENs. GR expression analysis at day 3 demonstrated a 50% decrease of GR protein, whereas the CD8+GR-Ex2 T cells engrafted in NSG mice treated with DEX or not did not express GR (Figure 5C). Overall, the results demonstrate not only the ability to render polyclonal T-cell populations resistant to glucocorticoids in vivo but also the need to ensure that clinical development focuses on generating highly pure populations of virus-specific T cells that are devoid of alloreactive potential for application in the transplant setting.
Resistance to DEX and functional activity of TALEN-modified CD8+ T cells in a xeno-GVHD model. (A) Hair loss (top row) and skin infiltration with human CD45+ cells counterstained with hematoxylin (middle row; inset shown in bottom row) from irradiated NSG mice injected with 1 × 106 CD8+wt or CD8+GR-Ex2 T cells and treated daily or not with intraperitoneally injected DEX at 15 mg/kg for 21 days. (B) Absolute number of human GZB-expressing (GZB+) CD8+wt or CD8+GR-Ex2 T cells in the spleens of previously described NSG mice. (C) Western blot analysis of GR expression from human-selected cells in pooled spleens ex vivo and at day 3 after transfection with TALENs. Data are representative means from 2 independent experiments with 5 mice per group; statistical analysis was performed by 1-way ANOVA (***P < .001) with Bonferroni multiple comparisons.
Resistance to DEX and functional activity of TALEN-modified CD8+ T cells in a xeno-GVHD model. (A) Hair loss (top row) and skin infiltration with human CD45+ cells counterstained with hematoxylin (middle row; inset shown in bottom row) from irradiated NSG mice injected with 1 × 106 CD8+wt or CD8+GR-Ex2 T cells and treated daily or not with intraperitoneally injected DEX at 15 mg/kg for 21 days. (B) Absolute number of human GZB-expressing (GZB+) CD8+wt or CD8+GR-Ex2 T cells in the spleens of previously described NSG mice. (C) Western blot analysis of GR expression from human-selected cells in pooled spleens ex vivo and at day 3 after transfection with TALENs. Data are representative means from 2 independent experiments with 5 mice per group; statistical analysis was performed by 1-way ANOVA (***P < .001) with Bonferroni multiple comparisons.
Discussion
We demonstrate efficient and rapid inactivation of the GR gene in CMV-specific T cells using electroporation of TALEN mRNA, leading to glucocorticoid resistance but retention of functionality in both in vitro assays and in vivo models. The processes described here are compatible with scaling to Current Good Manufacturing Practice regulatory standards, providing a potential strategy for treating patients with CMV infection and concurrent glucocorticoid therapy after HSCT. The transient expression of TALENs after mRNA electroporation offers a potential advantage for clinical applications, because the system does not require integration of the delivery vector, avoiding perceived risks associated with insertional mutagenesis.49 Furthermore, the process has already been established under clinical-scale Current Good Manufacturing Practice conditions.50 Transient expression also reduces the risks of off-target gene modification. For the GR-Ex2 TALENs, the 14 most likely off-target sites were identified by in silico analysis, and deep-sequencing analysis confirmed the high specificity of cleavage. Although this work focuses on the use of TALENs, other strategies for genome engineering such as (clustered regularly interspaced short palindromic repeat/clustered regularly interspaced short palindromic repeat–associated protein 9) could be applied in a similar fashion. Future studies may directly compare these technologies in terms of relative benefits with respect to clinical application. The specificity of targeting remains critical, and this is highly dependent on the design of targeting moieties. It is possible that no single technology will be superior for all applications.
Beyond the immediate translational application outlined for CMV, the approach could prove beneficial in other settings in which T-cell therapies are considered, because glucocorticoids are commonly used in medical practice. Applications for other viral infections such as adenovirus and Epstein-Barr virus that cause a significant burden of disease after HSCT in patients receiving corticosteroids are logical extensions, because adoptive T-cell therapies targeting these pathogens have demonstrated sustained antiviral efficacy and clinical benefits in patients not receiving corticosteroids.51,52 Particular consideration must be given to product purity in the setting of GVHD post-HSCT. Although CMVGR-Ex2 T cells would have a selective survival advantage over any remaining CMVwt T cells, the presence of alloreactive GR-deleted T cells would be a potential safety concern. This potential is highlighted by our results in the murine xeno-GVHD model. In the case of CMV, the use of a Streptamer-selection strategy allows rapid selection of highly pure populations of antigen-specific T cells. Attaining such high purities of other virus-specific T cells, such as adenovirus-specific T cells, remains more challenging. Because of these considerations, incorporation of a suicide gene may be justified in these translational applications.
Cell persistence will be important for clinical efficacy. This issue cannot be addressed definitively in a xenogeneic model in which longer-term engraftment of human cells is not adequately modeled,53 and will be most readily resolved within the context of a clinical trial. Memory phenotype of the infused cells may influence persistence. The recent demonstration that the distribution between naïve, effector, and memory subsets of double knockout chimeric antigen receptor (CAR)-modified T cells were indistinguishable from unmodified cells54 suggests that TALENs can efficiently target all of these T-cell subpopulations.
The strategy may also be applicable to T cell–based anticancer therapies. Many glioma and glioblastoma tumor cells express potential targets for CAR-based therapies, such as interleukin-13 receptor α2 or epidermal growth factor receptor variant III.55 Clinical application of CAR T cells is, however, hampered by the fact that patients with these malignancies receive glucocorticoids to reduce inflammation and cerebral edema after tumor resection. Glucocorticoids are also used as part of primary therapy in the clinical management of several other solid tumors, either because of their effectiveness in treating the malignancy56,57 or for managing tumor-associated symptoms (such as pain or edema).58 In all of these examples, GR resistance would both protect adoptively transferred cells from the effects of glucocorticoids and provide a selective survival advantage over endogenous T cells.
In conclusion, we show that glucocorticoid-resistant CMV-specific T cells can be rapidly generated in vitro using GR-TALENs and these GR gene–inactivated T cells maintain a specific cytolytic activity in the presence of DEX. Furthermore, when transferred into NSG recipients, TALEN-modified CD8+ T cells retained functionality and induced xeno-GVHD in the presence of DEX, providing evidence for their functional activity and resistance in vivo. Our findings have important implications for the transplantation field, because they represent the first proof of concept for the development of improved adoptive immunotherapy, using targeted genome modifications in T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ida Ricciardelli (Institute of Child Health, University College London [UCL]) for provision of B95-8 virus supernatant; James Motta (Universidad Andres Bello, Chile) for optimizing the immunohistochemical staining; the UCL Core Facility Cancer Genome Engineering Facility; Catherine King; Bill Lyons Informatics Centre; Herrero Javier; UCL/UCL Hospitals BioBank for Health and Disease; and UCL Biological Services.
This work was supported by a Cancer Research United Kingdom Career Development Fellowship and a Cancer Research Institute Investigator Award (S.A.Q); and Cancer Research United Kingdom, Leukaemia and Lymphoma Research, and the National Institute for Health Research Blood and Transplant Unit for stem cell transplantation and immunotherapy (K.S.P.). This work was undertaken at UCL/UCL Hospitals, which received support from the Department of Health and Cancer Research United Kingdom funding schemes for National Institute for Health Research Biomedical Research Centres and Experimental Cancer Medicine Centres.
Authorship
Contribution: L.M., M.A.V.M., K.B., and A.G. performed the experiments; L.M. analyzed the results; A.G. and J.S. provided TALEN GR and TALEN antibody; L.M., S.A.Q., and K.S.P. designed the research; and L.M. wrote the manuscript with input and approval from all the coauthors.
Conflict-of-interest disclosure: A.G. and J.S. are Cellectis employees. The remaining authors declare no competing financial interests.
Correspondence: Karl S. Peggs, Department of Haematology, University College London Cancer Institute, Paul O’Gorman Building, 72 Huntley St, London WC1E 6DD, United Kingdom; e-mail: karl.peggs@ucl.ac.uk; and Sergio A. Quezada, Department of Haematology, University College London Cancer Institute, Paul O’Gorman Building, 72 Huntley St, London WC1E 6DD, United Kingdom; e-mail: s.quezada@ucl.ac.uk.
References
Author notes
S.A.Q. and K.S.P. contributed equally as senior coauthors.