In this issue of Blood, Donato et al report that treatment of venous thromboembolism (VTE) with anticoagulation does not increase the risk of intracranial hemorrhage (ICH) in patients with solid tumors metastatic to the brain.1
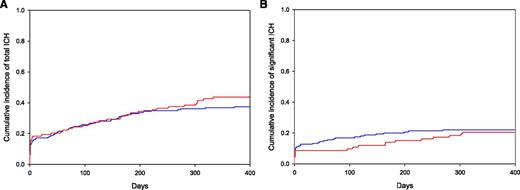
Cumulative incidence of ICH in patients with metastatic brain tumors. No difference in (A) the cumulative incidence of total and (B) significant ICH (>10 mL, symptomatic, or necessitating neurosurgery) was found in cancer patients with brain metastases when the control group (blue) was compared with patients treated with enoxaparin (red). See Figure 1B-C in the article by Donato et al that begins on page 494.
Cumulative incidence of ICH in patients with metastatic brain tumors. No difference in (A) the cumulative incidence of total and (B) significant ICH (>10 mL, symptomatic, or necessitating neurosurgery) was found in cancer patients with brain metastases when the control group (blue) was compared with patients treated with enoxaparin (red). See Figure 1B-C in the article by Donato et al that begins on page 494.
During lectures about anticoagulation, an image of a teeter-totter is often presented with bleeding on one side balanced by thrombosis on the other. The patient’s age, genetic conditions, medications, and comorbidities stack on either end to influence whether the balance is tipped toward bleeding or clotting. The width of the fulcrum and resulting fluctuation depend upon the clinical scenario. In hospital, mortality of patients with anticoagulation-associated ICH exceeds 30%2 ; thus, it is rare to find a situation more difficult to balance than anticoagulation in patients with brain metastases.
Because of the high risk of hemorrhage, the number of patients with brain lesions enrolled in randomized anticoagulation therapy trials has been limited. The pivotal CLOT trial established superiority of low-molecular-weight heparin over warfarin for VTE treatment in cancer patients; however, only 27 patients with primary brain tumors were included.3 Less than 1200 cancer patients have been enrolled in trials comparing the direct oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) to warfarin. The reports did not include the location of metastatic disease. Cancer patients enrolled in the direct oral anticoagulant trials were highly selected, though, and had a lower risk of recurrent thrombosis compared with patients enrolled in studies of low-molecular-weight heparin.4 Due to the paucity of prospective data, information about the safety of anticoagulation in patients with brain metastases must be gleaned from well-designed retrospective studies.
Excluding studies of primary brain tumors, 3 retrospective studies have evaluated the risk of anticoagulation in patients with brain metastases. No ICH occurred in 38 patients with tumors metastatic to brain treated with anticoagulation for a median of 13 weeks, but only 1 patient received therapeutic anticoagulation.5 Alvarado and colleagues reported outcomes of 81 patients with VTE and melanoma metastatic to brain.6 Of the 17 patients managed without anticoagulation, no one experienced an ICH compared with 4% of patients (2 of 57) treated with anticoagulation (P = 1.0). However, median follow-up was only 3.4 months.6 Donato and colleagues improved upon the flaws of the previous studies by designing their retrospective cohort study with a matched control group, blinded radiology review, and sophisticated statistical analysis using competing risk models.1 Cases were matched via a computer algorithm based on tumor type, year at diagnosis, age, and sex. ICH was subdivided based on symptoms and hemorrhage volume. Of the 104 patients treated with enoxaparin, 89% received therapeutic anticoagulation and 60% initiated anticoagulation after radiation or surgical treatment of the metastases. The 1-year cumulative incidence of all ICH was 44% in the enoxaparin cohort compared with 37% in the control cohort (see figure). Significant ICH, defined as a volume >10 mL, neurologic symptoms, or requiring surgical intervention, also did not differ between controls and patients treated with enoxaparin (1-year cumulative incidence of 22% vs 21%, respectively). Patients with renal cell carcinoma or melanoma were at an extraordinarily high risk of ICH (1-year cumulative incidence of 34% for significant bleeds and 58% for all ICH), which did not differ from patients treated with enoxaparin (35% for significant ICH and 55% for all ICH). Improvements in imaging and significantly longer follow-up could explain the higher incidence of ICH in this study.
Despite the well-designed study, its retrospective nature creates inherent limitations. The enoxaparin cohort included patients who were deemed eligible for anticoagulation. Theoretically, providers’ clinical acumen could have identified cancer patients with a lower risk of ICH in whom enoxaparin increased to the level of patients whom providers considered ineligible for anticoagulation. Unfortunately, besides tumor type, the multivariable analysis did not identify other clinical factors to guide clinicians in the assessment of ICH risk. Additionally, only 60 patients with renal cell carcinoma and melanoma (20 treated with enoxaparin and 40 controls) were available; thus, firm conclusions in this high-risk cohort cannot be established. Recognizing these limitations, the data presented suggest that anticoagulation does not increase the risk of ICH in patients with brain metastasis. Replicating this analysis in larger data sets and including patients with brain metastases in prospective studies are warranted.
Although oncologists often make the decisions regarding anticoagulation in cancer patients, hematologists are frequently consulted in scenarios involving a tenuous balance between bleeding and thrombosis. The results presented by Donato and colleagues suggest that cancer patients with brain metastasis have a wider fulcrum than expected to balance the risks of anticoagulation.1 This study further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin.7
Conflict-of-interest disclosure: The author declares no competing financial interests.