Key Points
Significant intracranial hemorrhage occurs in 20% to 50% of patients with metastatic brain tumors.
Therapeutic anticoagulation in patients with brain metastasis did not increase the risk for intracranial hemorrhage.
Abstract
Venous thromboembolism occurs frequently in patients with cancer who have brain metastases, but there is limited evidence supporting the safety of therapeutic anticoagulation. To assess the risk for intracranial hemorrhage associated with the administration of therapeutic doses of low-molecular-weight heparin, we performed a matched, retrospective cohort study of 293 patients with cancer with brain metastases (104 with therapeutic enoxaparin and 189 controls). A blinded review of radiographic imaging was performed, and intracranial hemorrhages were categorized as trace, measurable, and significant. There were no differences observed in the cumulative incidence of intracranial hemorrhage at 1 year in the enoxaparin and control cohorts for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages. The risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P < .001) in patients with melanoma or renal cell carcinoma (N = 60) than lung cancer (N = 153), but the risk was not influenced by the administration of enoxaparin. Overall survival was similar for the enoxaparin and control cohorts (8.4 vs 9.7 months; Log-rank, P = .65). We conclude that intracranial hemorrhage is frequently observed in patients with brain metastases, but that therapeutic anticoagulation does not increase the risk for intracranial hemorrhage.
Introduction
Approximately 20% of patients with brain metastases develop venous thromboembolism (VTE). However, with limited published evidence regarding the safety of anticoagulation in this population, the decision to prescribe therapeutic anticoagulation is challenging.1,2 Relatively few patients with brain metastasis have been enrolled in anticoagulant clinical trials. Among patients without cancer, the case-fatality rate for intracranial hemorrhage in the setting of anticoagulation often exceeds 30%.3,4 In the CLOT trial, which established the efficacy of low-molecular-weight heparin in the treatment of malignancy-associated VTE, 2 of 27 patients with brain tumors developed intracranial bleeding complications.5 Caution in prescribing anticoagulation in the setting of brain metastases is justified based on the high rate of spontaneous intracranial hemorrhage, especially in certain tumor types such as melanoma or renal cell carcinoma.6-9 Whether therapeutic anticoagulation further increases the risk for intracranial hemorrhage is unknown. We performed a control-matched cohort study of patients with brain metastases to determine whether the administration of therapeutic enoxaparin is associated with an increased risk for hemorrhage.
Methods
Study design
The protocol was approved by the institutional review board at the Dana-Farber Harvard Cancer Center. A 1:2 matched cohort study was performed at Beth Israel Deaconess Medical Center using electronic health record data from 1997 to 2014, which contains International Classification of Diseases, 9th Revision, diagnosis codes and prescription medication history.10 Enoxaparin cases were initially identified on the basis of coding for brain metastases, VTE, and prescription of enoxaparin. Matched controls for each case were identified using a “round-robin” scoring algorithm that ranked controls according to tumor diagnosis, year of diagnosis of brain metastasis, age, and gender. Each enoxaparin case was successfully matched with at least 1 cancer control and, if available, a second control. Inclusion criteria for the enoxaparin cases included metastatic solid tumor malignancy, parenchymal central nervous system (CNS) metastatic tumor by radiologic imaging, and therapeutic enoxaparin after the diagnosis of CNS metastasis for the treatment of VTE. Patients were excluded for primary brain tumors or leptomeningeal disease alone, nonsolid tumors (eg, lymphoma), absence of follow-up CNS imaging, or therapeutic anticoagulation (in the control group). A manual review of the online medical record was performed to ensure eligibility.
Intracranial hemorrhage
All available radiology reports (computed tomography of the head and magnetic resonance imaging of the brain) were reviewed for documentation of hemorrhage or blood products. The clinical indication and subsequent management of all documented intracranial hemorrhages were collected from the online medical record. The primary radiology images of all intracranial hemorrhages were re-reviewed by a neurooncologist blinded to cohort allocation (enoxaparin versus control) to confirm the presence of intracranial hemorrhage and to calculate the volume of blood using the one-half ABC technique.11,12 Measurable intracranial hemorrhages were those defined as greater than 1 mL in volume, whereas trace intracranial hemorrhages were those defined as less than 1 mL in volume. Total hemorrhages included both measurable and trace hemorrhages. Each hemorrhage (trace and measurable) was further classified as a “significant” intracranial hemorrhage on the basis of predetermined criteria; significant intracranial hemorrhages were those defined as greater than 10 mL in volume, symptomatic (defined as focal neurologic deficit, headache, nausea, or change in cognitive function), or required surgical intervention.12-14 All demographic and endpoint information was entered into a RedCap database maintained by the Harvard Catalyst program.
Statistical analysis
The primary endpoint of the study was measurable intracranial hemorrhage from initial diagnosis of brain metastases. Initial sample size estimates were based on an anticipated rate of intracranial hemorrhage of 7% in the enoxaparin cohort and 2% in the control cohort.6,7,15 Accordingly, the target analysis was approximately 200 patients treated with enoxaparin and 400 controls (1-sided α of 0.05% and power of 0.85). For the 3 primary bleeding end points, analysis was performed using a competing risk approach to account for death as a competing risk for intracranial hemorrhage.16 Estimations of the cumulative incidence of measurable, significant, and total intracranial hemorrhages from initial diagnosis of brain metastases were calculated using the competing risk analysis, with significance testing performed using a the Gray test. Estimations of hazard ratio (HR) of event rates between the enoxaparin cohort and controls were performed using the Fine and Gray competing risk regression model. In addition, to study the effect of enoxaparin on the cumulative incidence of measurable intracranial hemorrhage, we considered a Fine and Gray regression in which a time-varying covariate was created to indicate the moment when patients in the enoxaparin cohort started treatment. Because patients treated with enoxaparin started treatment during the course of the observational study, rather than at study onset, the time-dependent covariate represented the change in enoxaparin status for this cohort of patients. Overall survival (OS) was estimated using the method of Kaplan and Meier, and the log-rank test was used to compare OS between groups. OS was defined as time from diagnosis of brain metastases to date of death from any cause. Patients who were still alive were censored at date of last known contact. Comparisons between groups were performed using the Fisher exact test for binary end points. A 2-sided P value less than 5% was considered statistically significant. Landmark analysis of overall survival was performed using a Cox regression, including patients alive at 6 months and excluding patients who did not yet initiate enoxaparin.
Results
A total of 293 patients with confirmed brain metastases were included in the study: 104 patients in the enoxaparin cohort and 189 patients in the control cohort (Table 1). The predominant cancer subgroup was non-small cell lung cancer, followed by breast cancer, renal cell carcinoma, and melanoma. The 2 cohorts were well matched for tumor diagnosis, age, sex, gender, comorbidities, number of CNS metastases, and types of treatment of the CNS metastasis (eg, surgery, radiation, or chemotherapy). The use of aspirin was significantly higher in the control cohort than in the enoxaparin cohort (15.3% vs 4.8%; Fisher’s exact P = .007), presumably as a result of prescriber caution in administering dual antithrombotic therapy (aspirin and anticoagulant). Enoxaparin was administered twice daily (1 mg/kg) to 76 patients (73.1%), once daily (1.5 mg/kg) to 17 patients (16.3%), and at modified (dose-reduced) therapeutic dosing to 11 patients in a setting of thrombocytopenia or renal failure. In the majority of cases, enoxaparin was initiated after brain metastases were diagnosed (88 of 104; 84.6%).
Demographics and patient characteristics
| Characteristic . | Enoxaparin (N = 104) . | Controls (N = 189) . |
|---|---|---|
| Males, n (%) | 55 (52.9%) | 94 (49.7%) |
| Mean age at time of brain metastasis, y (range) | 60.9 (31.1-84.6) | 60 (21.9-92.1) |
| Stage 4 at time of cancer diagnosis, n (%) | 46 (44.2%) | 91 (48.1%) |
| Number of brain lesions when first recognized, n (%) | ||
| 1-2 | 63 (60.6%) | 107 (56.6%) |
| 3-4 | 10 (9.6%) | 29 (15.3%) |
| 5 or more | 16 (15.4%) | 25 (13.2%) |
| Primary malignancy, n (%) | ||
| Non-small cell lung cancer | 56 (53.8%) | 97 (51.3%) |
| Breast cancer | 12 (11.5%) | 25 (13.2%) |
| Renal cell carcinoma | 10 (9.6%) | 20 (10.6%) |
| Melanoma | 10 (9.6%) | 20 (10.6%) |
| Colorectal cancer | 5 (4.8%) | 9 (4.8%) |
| Small cell lung cancer | 2 (1.9%) | 6 (3.2%) |
| Comorbidities, n (%) | ||
| Hypertension | 40 (38.5%) | 76 (40.2%) |
| Chronic kidney disease | 5 (4.8%) | 18 (9.5%) |
| Treatment of brain metastasis, n (%) | ||
| Chemotherapy after brain met diagnosis | 72 (69.2%) | 115 (60.8%) |
| Brain radiation* | 82 (78.8%) | 163 (86.2%) |
| Neurosurgery | 30 (28.8%) | 44 (23.3%) |
| Corticosteroids for cerebral edema | 74 (71.2%) | 162 (85.7%) |
| Neurosurgery or brain radiation | 83 (79.8%) | 168 (88.9%) |
| Concomitant medications | ||
| Aspirin use, n (%) | 5 (4.8%) | 29 (15.3%)† |
| Antiangiogenic agents | 14 (13.5%) | 10 (5.2%)‡ |
| Characteristic . | Enoxaparin (N = 104) . | Controls (N = 189) . |
|---|---|---|
| Males, n (%) | 55 (52.9%) | 94 (49.7%) |
| Mean age at time of brain metastasis, y (range) | 60.9 (31.1-84.6) | 60 (21.9-92.1) |
| Stage 4 at time of cancer diagnosis, n (%) | 46 (44.2%) | 91 (48.1%) |
| Number of brain lesions when first recognized, n (%) | ||
| 1-2 | 63 (60.6%) | 107 (56.6%) |
| 3-4 | 10 (9.6%) | 29 (15.3%) |
| 5 or more | 16 (15.4%) | 25 (13.2%) |
| Primary malignancy, n (%) | ||
| Non-small cell lung cancer | 56 (53.8%) | 97 (51.3%) |
| Breast cancer | 12 (11.5%) | 25 (13.2%) |
| Renal cell carcinoma | 10 (9.6%) | 20 (10.6%) |
| Melanoma | 10 (9.6%) | 20 (10.6%) |
| Colorectal cancer | 5 (4.8%) | 9 (4.8%) |
| Small cell lung cancer | 2 (1.9%) | 6 (3.2%) |
| Comorbidities, n (%) | ||
| Hypertension | 40 (38.5%) | 76 (40.2%) |
| Chronic kidney disease | 5 (4.8%) | 18 (9.5%) |
| Treatment of brain metastasis, n (%) | ||
| Chemotherapy after brain met diagnosis | 72 (69.2%) | 115 (60.8%) |
| Brain radiation* | 82 (78.8%) | 163 (86.2%) |
| Neurosurgery | 30 (28.8%) | 44 (23.3%) |
| Corticosteroids for cerebral edema | 74 (71.2%) | 162 (85.7%) |
| Neurosurgery or brain radiation | 83 (79.8%) | 168 (88.9%) |
| Concomitant medications | ||
| Aspirin use, n (%) | 5 (4.8%) | 29 (15.3%)† |
| Antiangiogenic agents | 14 (13.5%) | 10 (5.2%)‡ |
Antiangiogenic agents include bevacizumab, sorafenib, or sunitinib. CNS, central nervous system; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Whole-brain radiation or stereotactic surgery.
Fishers exact, P value = .007.
P = .06.
Cumulative incidence of intracranial hemorrhage
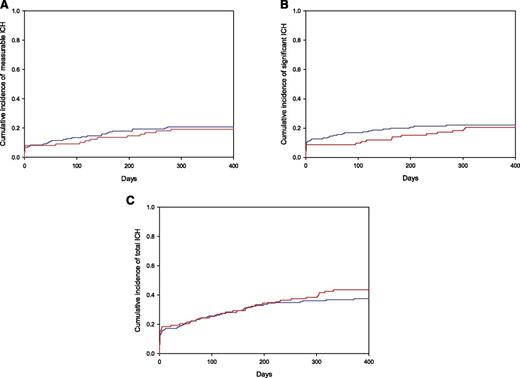
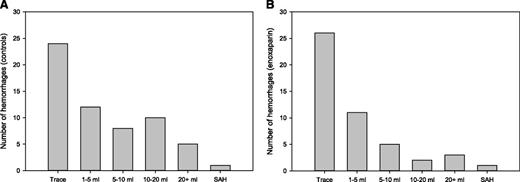
There was no statistical difference in the cumulative incidence of measurable, total, or significant intracranial hemorrhage in those patients who received therapeutic enoxaparin after the diagnosis of brain metastases compared with matched controls who did not receive therapeutic anticoagulation. As shown in Figure 1, the cumulative incidence of measurable intracranial hemorrhages at 1 year was 19% in the enoxaparin cohort and 21% in the control cohort (Gray test, P = .97; HR, 1.02; 90% confidence interval [CI], 0.66-1.59). The cumulative incidence of total intracranial hemorrhages was 44% at 1 year in the enoxaparin cohort compared with 37% in the control cohort (P = .13). As shown in the histogram in Figure 2, the volume distribution of the intracranial hemorrhages was similar in the 2 cohorts. There were a similar number of radiologic studies performed before the date of intracranial hemorrhage, loss to follow-up, or death in the enoxaparin and control cohorts (median of 4 vs 3 studies; P = .12).
Cumulative incidence of intracranial hemorrhage (ICH) in patients with metastatic brain tumors. No differences between enoxaparin and control cohorts were observed in the cumulative incidence of intracranial hemorrhage for any category (Gray test, P > .05) including measurable (A), significant (B), and total (C) hemorrhages. Enoxaparin cohort shown in red and controls in blue.
Cumulative incidence of intracranial hemorrhage (ICH) in patients with metastatic brain tumors. No differences between enoxaparin and control cohorts were observed in the cumulative incidence of intracranial hemorrhage for any category (Gray test, P > .05) including measurable (A), significant (B), and total (C) hemorrhages. Enoxaparin cohort shown in red and controls in blue.
Volume histograms of intracranial hemorrhages. The distribution of all hemorrhages identified among control (A) and enoxaparin (B) cohorts are shown according to volume (mL). SAH, subarachnoid hemorrhage.
Volume histograms of intracranial hemorrhages. The distribution of all hemorrhages identified among control (A) and enoxaparin (B) cohorts are shown according to volume (mL). SAH, subarachnoid hemorrhage.
An intracranial hemorrhage was identified as significant on the basis of size, symptoms, and/or need for surgical intervention. We did not detect a difference in the rate of significant intracranial hemorrhage between the groups who received enoxaparin compared with controls, with a 1-year cumulative incidence of significant intracranial hemorrhage of 21% in the enoxaparin cohort compared with 22% in the control cohort (Gray test, P = .87). The majority of significant intracranial hemorrhages in both the enoxaparin (21 of 24; 87.5%) and the control cohorts (36 of 38; 94.7%) were symptomatic. Six of 24 patients (25%) in the enoxaparin cohort with significant hemorrhages required neurosurgical intervention compared with 6 of 38 (15.8%; Fisher’s exact test, P = .51) in the control group.
Intracranial hemorrhage according to malignancy diagnosis
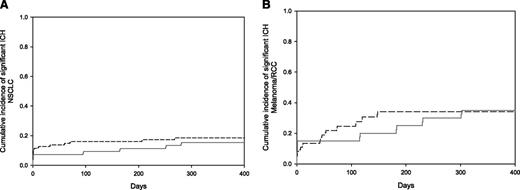
Among those patients with nonsmall cell lung cancer (N = 153), there was no statistical difference in the cumulative incidence of measurable, total, or significant intracranial hemorrhages. The cumulative incidence of intracranial hemorrhage at 1 year in the enoxaparin cohort compared with the control cohort was 12% vs 16% for measurable bleeds (Gray test, P = .5), 15% vs 19% for significant bleeds (P = .93), and 42% vs 33% for total bleeds (P = .23), as shown in Figure 3. Among those patients with melanoma or renal cell carcinoma (N = 60), the overall rate of intracranial hemorrhage was considerably greater (Table 2) than other malignancy subgroups, but there was no statistical difference in the incidence of intracranial hemorrhages in the enoxaparin compared with the control cohorts within the subgroup. In the melanoma plus renal cell carcinoma subgroup, the cumulative incidence of intracranial hemorrhage at 1 year in the enoxaparin cohort was 42% compared with 52% in the control cohort for measurable bleeds (Gray test, P = .8), 35% vs 34% for significant bleeds (P = .88), and 55% vs 58% for total bleeds (P = .75) (Figure 3).
Cumulative incidence of significant intracranial hemorrhage (ICH) in the non-small cell lung cancer and melanoma/renal cell carcinoma subgroups. (A) The cumulative incidence of significant intracranial hemorrhage in patients with non-small lung cancer at 1 year was 15% in the enoxaparin cohort compared with 19% in the control cohort (Gray test, P = .93). (B) In the melanoma plus renal cell carcinoma subgroup, the cumulative incidence of significant intracranial hemorrhage at 1 year was 35% for the enoxaparin cohort vs 34% for the controls (Gray test, P = .88). Enoxaparin cohort shown in solid gray line and controls in hatched black line. NSCLC, nonsmall lung cancer; RCC, renal cell carcinoma.
Cumulative incidence of significant intracranial hemorrhage (ICH) in the non-small cell lung cancer and melanoma/renal cell carcinoma subgroups. (A) The cumulative incidence of significant intracranial hemorrhage in patients with non-small lung cancer at 1 year was 15% in the enoxaparin cohort compared with 19% in the control cohort (Gray test, P = .93). (B) In the melanoma plus renal cell carcinoma subgroup, the cumulative incidence of significant intracranial hemorrhage at 1 year was 35% for the enoxaparin cohort vs 34% for the controls (Gray test, P = .88). Enoxaparin cohort shown in solid gray line and controls in hatched black line. NSCLC, nonsmall lung cancer; RCC, renal cell carcinoma.
Univariable and multivariable Fine and Gray competing risk analysis for the development of measurable intracranial hemorrhage
| . | Hazard ratio (90% CI) . | P value . |
|---|---|---|
| Univariable regression | ||
| Age | 1.01 (0.99-1.03) | .39 |
| Enoxaparin | 1.02 (0.66-1.59) | .93 |
| Primary malignancy (Lung cancer as reference) | ||
| RCC/Melanoma | 4.03 (2.56-6.34) | <.001 |
| Breast cancer/colorectal cancer/Other | 0.62 (0.29-1.33) | .30 |
| Hypertension | 0.84 (0.53-1.33) | .54 |
| Chronic kidney disease | 3.19 (1.75-5.85) | .002 |
| Aspirin use | 0.68 (0.32-1.45) | .40 |
| More than 1 brain met at time of diagnosis | 0.99 (0.61-1.59) | .97 |
| Antiangiogenic agents | 0.89 (0.41-1.96) | .81 |
| Multivariable regression | ||
| Primary malignancy (lung cancer as reference) | ||
| RCC/melanoma | 3.98 (2.41-6.57) | <.001 |
| Breast cancer/colorectal cancer/Other | 0.66 (0.31-1.41) | .36 |
| Chronic kidney disease | 1.62 (0.82-3.16) | .24 |
| . | Hazard ratio (90% CI) . | P value . |
|---|---|---|
| Univariable regression | ||
| Age | 1.01 (0.99-1.03) | .39 |
| Enoxaparin | 1.02 (0.66-1.59) | .93 |
| Primary malignancy (Lung cancer as reference) | ||
| RCC/Melanoma | 4.03 (2.56-6.34) | <.001 |
| Breast cancer/colorectal cancer/Other | 0.62 (0.29-1.33) | .30 |
| Hypertension | 0.84 (0.53-1.33) | .54 |
| Chronic kidney disease | 3.19 (1.75-5.85) | .002 |
| Aspirin use | 0.68 (0.32-1.45) | .40 |
| More than 1 brain met at time of diagnosis | 0.99 (0.61-1.59) | .97 |
| Antiangiogenic agents | 0.89 (0.41-1.96) | .81 |
| Multivariable regression | ||
| Primary malignancy (lung cancer as reference) | ||
| RCC/melanoma | 3.98 (2.41-6.57) | <.001 |
| Breast cancer/colorectal cancer/Other | 0.66 (0.31-1.41) | .36 |
| Chronic kidney disease | 1.62 (0.82-3.16) | .24 |
Variables predictive of intracranial hemorrhage
Univariate Fine and Gray competing risk regression was performed to identify variables predictive of measurable intracranial hemorrhage in patients with metastatic brain tumors (Table 2). Among the variables analyzed, both malignancy diagnosis (melanoma or renal cell carcinoma) and chronic kidney disease were predictive of measurable intracranial hemorrhage. In a Fine and Gray competing risk regression, the strongest predictor of intracranial hemorrhage was the combined tumor category of melanoma and renal cell carcinoma, which was associated with an almost fourfold increased risk for measurable intracranial hemorrhage (HR, 3.98; 90% CI, 2.41-6.57) relative to lung cancer. The cumulative incidence of intracranial hemorrhage did not differ statistically based on the dosing regimen of enoxaparin (once daily vs twice daily) for measurable (Gray’s test, P = .20), significant (P = .91), or total (P = .46) intracranial hemorrhages. The use of antiangiogenic agents (ie, bevacizumab, sorafenib, or sunitinib) was not associated with an increased risk for measurable intracranial hemorrhage (HR, 0.89; 90% CI, 0.41-1.96).
Intracranial hemorrhage relative to the initiation of enoxaparin
The cumulative incidence of measurable intracranial hemorrhage after the start of enoxaparin was 11.8% at 6 months. The cumulative incidence of measurable hemorrhage was 13% at 6 months in the subgroup (N = 62) when enoxaparin was initiated after receiving CNS-directed therapy (radiation or surgery), which addresses the frequent clinical question regarding risk for intracranial hemorrhage in patients with treated brain metastasis. We also considered enoxaparin as a time-varying covariate to assess the effect of anticoagulation on the cumulative incidence of intracranial hemorrhage. Treatment with enoxaparin was associated with a decreased incidence of measurable intracranial hemorrhage (HR, 0.47; 90% CI, 0.27-0.80; P = .02).
Overall survival
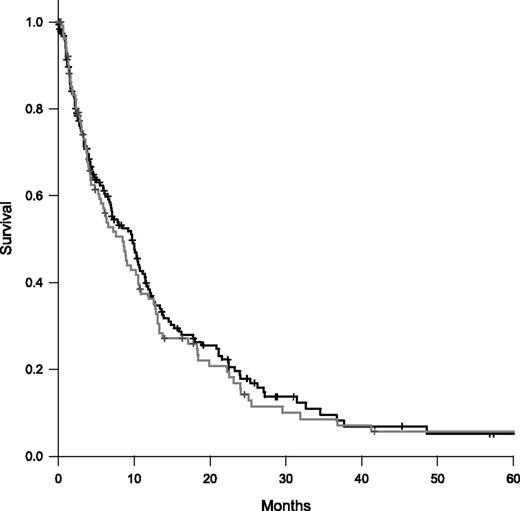
A Kaplan-Meier survival analysis was performed for the 2 cohorts. As shown in Figure 4, the median survival was similar in the enoxaparin and control cohorts (8.4 vs 9.7 months; Log-rank test, P = .65). We performed post hoc analysis to explore the potential of immortal time bias. The majority of patients in enoxaparin cohort initiated anticoagulation by 6 months (87 of 104, 83.7%). Of the 87 enoxaparin patients, 36 (41.4%) were alive at 6 months compared with 96 (52.2%) of 184 in the control cohort (Fishers exact, P = .12). In a landmark analysis of OS including patients alive at 6 months, the HR was 1.56 (95% CI, 1.01-2.41; P = .05).
Kaplan-Meier survival curves comparing enoxaparin and control cohorts. The median survival was 8.4 months in the enoxaparin cohort (gray) and 9.7 months in the control cohorts (black). Log-rank test, P = .65.
Kaplan-Meier survival curves comparing enoxaparin and control cohorts. The median survival was 8.4 months in the enoxaparin cohort (gray) and 9.7 months in the control cohorts (black). Log-rank test, P = .65.
Discussion
VTE is a common complication in patients with brain metastases, but limited evidence is available regarding whether therapeutic anticoagulation can be safely administered. To the best of our knowledge, this is the largest clinical study assessing the safety of low-molecular-weight heparin in patients with metastatic brain tumors. Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages.
The absence of an effect of low-molecular-weight heparin on rates of intracranial hemorrhage is consistent with the findings of smaller studies, including a case series of 38 patients15 and a retrospective cohort study of 44 patients with melanoma brain metastases.7 In the current study, the only covariate that was predictive of hemorrhage was the combined group of renal cell carcinoma and melanoma. This finding is consistent with published data regarding the high incidence of spontaneous intracranial hemorrhage associated with both melanoma and renal cell carcinoma.17 The diagnosis of brain metastasis secondary to melanoma and renal cell carcinoma is commonly considered a relative contraindication for therapeutic anticoagulation.1,2 Despite an intracranial hemorrhage incidence of nearly 50% at 1 year in the melanoma and renal cell carcinoma group, we did not observe an additional risk for intracranial hemorrhage attributed to the use of low-molecular-weight heparin. However, on the basis of the high rate of intracranial hemorrhage and the limited number of patients with such diagnoses in our cohort study, caution is warranted in treating patients with melanoma and renal cell carcinoma with therapeutic anticoagulation.
Even in the absence of anticoagulation, brain metastases are associated with high rates of spontaneous hemorrhage. The rates observed in this study are higher than previously reported, which is likely a result of increased sensitivity of modern imaging and longer follow-up in the current study, as well as differences in the definition of intracranial hemorrhage. We elected to use strict criteria for intracranial hemorrhage, using a radiologic estimation of hemorrhage volume. In contrast, most studies evaluating the rates of hemorrhage related to intracranial metastases do not provide a priori criteria to define intracranial hemorrhage2,6-9,13 The definition of a significant intracranial hemorrhage on anticoagulation also varies according to which classification criteria are applied.13,14 Intracranial blood volume is known to be predictive of poor outcomes in hemorrhagic stroke patients11,12 ; however, extrapolating similar blood volume thresholds to cancer cohorts is difficult because of the coexistent tumor mass. On the basis of the incomplete literature available to accurately describe the spectra of intracranial hemorrhages in patients with cancer, we elected to incorporate elements of several classification approaches into a composite “significant” intracranial hemorrhage category, which included larger-volume bleeds (>10 mL), the presence of new symptoms, or the need for surgical intervention. Notably, the vast majority of all bleeds classified into this category were symptomatic (>90%), and approximately one-quarter required surgical intervention.
Several limitations of the study warrant discussion. First, there are intrinsic limitations and biases inherent to retrospective cohort studies. Ideally, patients are identified at the time of CNS metastasis and are prospectively followed for the development of intracranial hemorrhage with or without anticoagulation. Such a study is difficult to perform logistically and is prone to imbalances, as patients are not randomly allocated to treatment groups. A specific strength of the current cohort study is the use of a computerized scoring algorithm to best match controls for a number of baseline characteristics. We also used a blinded review of radiology images to minimize classification bias. Although it is possible that those patients identified as appropriate for anticoagulation represent a lower-risk population, and thus experienced a comparatively lower rate of hemorrhage, we did not observe a significant difference in rates of hemorrhage between those who received anticoagulation compared with those who did not when anticoagulation was considered appropriate by the treating physician. We did not assess the factors influencing the decision to administer anticoagulation, and thus would continue to advocate for clinical judgment in determining the appropriateness of therapeutic anticoagulation for any given patient. Beyond malignancy diagnosis, we did not identify any independent risk factors (ie, renal failure, age, hypertension, etc) that would assist the clinician in determining the safety of anticoagulation. Whether other laboratory risk factors such as thrombocytopenia or baseline coagulopathy serve as predictors for hemorrhage was not assessed in the current study. The second potential limitation is that we did not achieve the target number of patients based on our original statistical assumptions. However, all eligible patients identified at a major academic teaching hospital between 1997 and 2014 were included, and we did not observe any trends toward an increased risk for intracranial hemorrhage associated with the administration of enoxaparin over time; on the basis of these results, a trial powered to detect such a small statistical difference between study groups would require several thousand patients.
Deep vein thrombosis in patients with cancer is associated with shortened survival relative to patients with cancer without VTE.18,19 Whether low-molecular-weight heparin affects overall survival in patients with cancer continues to be debated.20 A trend toward improved survival with low-molecular-weight heparin was observed in a smaller study of patients with melanoma with brain metastasis (median overall survival, 4.3 months vs 1.2 months; P = .06).7 In such cohort studies, there is the potential for immortal time bias, as the experimental group must live long enough to suffer the event of interest (ie, VTE). In our study, overall survival did not differ between the enoxaparin and control cohorts and was largely influenced by a similarly high rate of early mortality. In the subgroup of patients who lived past 6 months, a landmark analysis suggests the diagnosis of VTE potentially carries a worse long-term prognosis.
Given the high incidence of VTE and the potential for devastating intracranial hemorrhage with anticoagulation, it is important to provide evidence to help guide clinical decisions regarding the use of anticoagulation in patients with brain metastases. Recent guidelines issued by American Society of Clinical Oncology state that the presence of an intracranial malignancy should not be considered an absolute contraindication for anticoagulation.21 The data presented in this study provide reassurance that low-molecular-weight heparin can safely be administered to patients with metastatic brain tumors without increasing the likelihood of intracranial hemorrhage.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Computerized data collection was supported by the Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers). Statistical support provided through a Dana-Farber/Harvard Cancer Center Core Grant (5P30 CA006516).
Authorship
Contribution: The manuscript was principally authored by J.D. and J.I.Z. and reviewed by all authors; the study was designed by J.D., F.C., D.N., E.J.U., G.M.W., and J.I.Z.; and data collection and analysis were performed by all authors.
Conflict-of-interest disclosure: J.I.Z. received prior research funding from Sanofi, serving on advisory committees for Portola Pharmaceuticals and Merck, and receiving consulting fees from Parexel. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey Zwicker, Division of Hemostasis and Thrombosis, Beth Israel Medical Center, 330 Brookline Ave, Boston, MA 02115; e-mail: jzwicker@bidmc.harvard.edu.