Key Points
Wip1 controls antigen-independent B-cell development in the bone marrow via a p53-dependent pathway.
Wip1 is essential to prevent an aging-related decline in B-cell development.
Abstract
Wild-type p53-induced phosphatase 1 (Wip1), a phosphatase previously considered as an oncogene, has been implicated in the regulation of thymus homeostasis and neutrophil maturation. However, the role of Wip1 in B-cell development is unknown. We show that Wip1-deficient mice exhibit a significant reduction of B-cell numbers in the bone marrow, peripheral blood, and spleen. A reciprocal transplantation approach revealed a cell-intrinsic defect in early B-cell precursors caused by Wip1 deficiency. Further experiments revealed that Wip1 deficiency led to a sustained activation of p53 in B cells, which led to increased level of apoptosis in the pre–B-cell compartment. Notably, the impairment of B-cell development in Wip1-deficient mice was completely rescued by genetic ablation of p53, but not p21. Therefore, loss of Wip1 phosphatase induces a p53-dependent, but p21-independent, mechanism that impairs B-cell development by enhancing apoptosis in early B-cell precursors. Moreover, Wip1 deficiency exacerbated a decline in B-cell development caused by aging as evidenced in mice with aging and mouse models with serial competitive bone marrow transplantation, respectively. Our present data indicate that Wip1 plays a critical role in maintaining antigen-independent B-cell development in the bone marrow and preventing an aging-related decline in B-cell development.
Introduction
B-cell development in the bone marrow is a precisely ordered developmental process with multiple checkpoints after the rearrangement of immunoglobulin heavy- and light-chain gene loci.1 The successful V(D)J rearrangement in B cells is orchestrated by a series of complex molecular events including the activation of several transcription factors, like PU.1, E2a, Ebf, and Pax5.2-4 During the developmental process, B cells encounter multiple signaling regulations and various cell-fate decisions.5 Defined stages of committed B-cell precursors include pro–B cells, pre–B cells, and finally immature and mature B cells expressing variable amounts of surface immunoglobulin M (IgM) and other markers.6-8 Although studies on different mouse mutants provided fundamental insights into this process,7-9 the detailed molecular regulation mechanisms of early B-cell development are still poorly understood.
Wild-type (WT) p53-induced phosphatase 1 (Wip1, also called PP2Cδ or PPM1D) is a serine/threonine protein phosphatase belonging to the type 2Cδ protein phosphatases.10 It is activated by various stresses and involved in various cellular processes such as tumorigenesis and aging.11-13 Wip1 is recognized as a novel oncogene and is widely believed to be a promising therapeutic target for cancers.14,15 The roles of Wip1 in the hematopoietic system recently caused much attention. Wip1 critically regulates granulocyte development and function via p38 mitogen-activated protein kinase/signal transducer and activator of transcription 1–dependent pathways.16-18 Wip1 has also been shown to be essential for the homeostasis of mature medullary thymic epithelial cells and the maturation of T cells in p53-dependent and independent manners.19,20 However, the roles of Wip1 in the regulation of B-cell development are still unknown, although it is known that deletion of Wip1 dramatically delays the onset of Eμ-myc–induced B-cell lymphomas via its inhibitory effect on the ataxia telangiectasia mutated kinase.21 In the present study, we used Wip1-deficient mice to investigate the roles of phosphatase Wip1 in B-cell development in the bone marrow. We found that Wip1 deficiency resulted in a significant impairment of antigen-independent B-cell development from hematopoietic stem and progenitor cells in a cell-intrinsic manner. Interestingly, this impaired B-cell development in Wip1-deficient mice occurs in early B-cell precursors, which can be completely rescued by genetic ablation of p53. Thus, this study revealed a novel function of phosphatase Wip1 in the positive regulation of B-cell development in the bone marrow through a p53-mediated pathway.
Materials and methods
Mice
Mice with a deficiency of Wip1 (Ppm1dtm1Lad), p21 (Cdkn1atm1Led), and p53 (Trp53tm1Tyj), respectively, have been previously described.22-25 Wip1 knockout (KO) mice were backcrossed to the C57BL/6 background in our laboratory.16 Wip1/p53 and Wip1/p21 double-knockout (DKO) mice were generated by crossing Wip1KO with p53KO or p21KO mice. Six- to 8-week-old female CD45.1 mice were purchased from Beijing University Experimental Animal Center (Beijing, China). All mice were maintained in a specific-pathogen–free facility. All experimental manipulations were undertaken in accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals, Institute of Zoology (Beijing, China).
Flow cytometry and cell sorting
Bone marrow cells (BMCs) isolated from femurs, tibiae, and iliac crests were isolated as reported previously.26 The BMCs were suspended in staining buffer (phosphate-buffered saline [PBS] supplemented with 2% fetal bovine serum). The following antibodies purchased from eBioscience or BioLegend: CD19 (eBio1D3), B220 (RA3-6B2), CD43 (eBioR2/60), IgM (11/41), CD45.1 (A20), and CD45.2 (104). The non–B-lineage cocktail was a mixture of the following antibodies: CD4 (RM4-5), CD8 (53-6.7), Ter-119 (TER-119), CD11b (M1/70), Gr-1 (RB6-8C5), NK1.1 (PK136), and CD11c (N418). Streptavidin was purchased from BD Biosciences. After staining, cells were suspended and maintained at 4°C before fluorescence activated cell sorter (FACS) analysis. Data acquisition was performed on a BD Fortessa. Cell sorting was conducted on BD Influx (Becton Dickson). The B-cell subtypes were identified by flow cytometry or sorted as follows: total B cells (B220+CD19+), pre-pro–B cells (B220+CD43+CD19−), pro–B cells (B220+CD43+CD19+), pre–B cells (B220midCD43−IgM−CD19+), immature B cells (B220midCD43−IgM+CD19+), and mature B cells (B220hiCD43−IgM+CD19+). Data were analyzed by FlowJo software.
Mixed bone marrow chimeras
Serial competitive bone marrow transplantation was performed by transplanting total BMCs (5 × 106) from 8-week-old WT and Wip1KO mice (donor, CD45.2) with 5 × 106 BMCs from young (2- to 4-month-old) competitor mice (CD45.1 or CD45.1/CD45.2) into lethally irradiated congenic mice (CD45.1).16 Donor-derived bone marrow mononuclear cells were collected from primary recipient mice after 12 weeks of transplantation and transplanted into secondary recipient mice with fresh competitor cells. The same procedure was used for the tertiary transplantation.
Genotyping primers
The Wip1, p53, and p21 genotypes were determined from tail DNA by polymerase chain reaction (PCR) using the following primers: Wip1 primer 1, GACAGTCCTGTGCCAAAATGCT; Wip1 primer 2, GGTGACTTGATTGGTGGTGTAGA; Wip1 primer 3, GCAGGGCTGTTTGTGGTGCT; Wip1 primer 4, GCATGCTCCAGACTGCCTT; p53 primer 1, GTGTTTCATTAGTTCCCCACCTTGAC; p53 primer 2, GGA GGCTGCCAGTCCTAAC; p53 primer 3, GTGGGAGGGACAAAAGTTCGAG; p53 primer 4, AGCCCTGGCGCTCGATGT; p21 primer 1, CATTCCAGCCCTTCCCCAC; p21 primer 2, CTTCAGGGTTTTCTCTTGCAG; and p21 primer 3, ATCGCCTTCTATCGCCTTCTTGACGAG.
Blood cell counts
For analysis of blood counts, peripheral blood from the postorbital vein was collected and analyzed on a hematology analyzer (Sysmex).
RNA purification and real-time PCR
Total RNA was isolated with TRIzol (Invitrogen), and reverse transcription was performed with M-MLV superscript reverse transcriptase according to the manufacturer’s instructions. Real-time PCR was performed using multiple kits (SYBR Premix Ex Taq, DRR041A; Takara Bio) on a CFX96 Real-Time System (Bio-Rad). The messenger RNA (mRNA) expression levels of each gene were normalized to the expression level of the housekeeping gene hypoxanthine phosphoribosyl transferase (Hprt) or β-actin (Actb) as indicated: p53 forward, CTCCCATGTGCTCAAGACTG; p53 reverse, TCGACGCTAGGATCTGACTG; Wip1 forward, CTGACTGATAGCCCTACTTACAACA; Wip1 reverse, GAGAAGGCATTACTGCGAACA; Hprt forward, AGTACAGCCCCAAAATGGTTAAG; Hprt reverse, CTTAGGCTTTGTATTTGGCTTTTC; Actb forward, GGCTGTATTCCCCTCCATCG; and Actb reverse, CCAGTTGGTAACAATGCCATGT.
Apoptosis and cell proliferation assays
For the apoptosis assay, BMCs were stained with antibodies for surface markers, washed with cold PBS, and incubated at room temperature for 15 minutes in 1× binding buffer (eBioscience) containing Annexin V–fluorescein isothiocyanate (eBioscience) and (4′,6 diamidino-2-phenylindole) DAPI (1 mg/mL) before analyzing by FACS. For the cell proliferation assay, BMCs were fixed and permeabilized with BD Cytofix/Cytoperm (BD Bioscience) after staining with monoclonal antibodies against cell-surface markers, then resuspended with PBS containing DAPI before analyzing by FACS.
Protein analyses
Total B cells in the bone marrow were sorted and lysed in ice-cold lysis buffer containing10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 2 mM EDTA (pH 8), 5 mM dithiothreitol, and 1 mM Pefabloc. Immunoblots were performed with the following antibodies: anti-phospho-p53 (ser15) and anti-β-actin monoclonal antibodies (Cell Signaling Technology and Sigma-Aldrich, respectively).
Statistical analysis
All data showed normal distribution (Shapiro-Wilk test, P > .05) and are presented as the mean ± standard deviation (SD). Two-way analysis of variance was used for comparison among multiple groups with SPSS 16.0 software. Student unpaired t test for comparison of means was used to compare between the 2 groups. P < .05 was considered statistically significant.
Results
Wip1KO mice exhibit a significantly decreased B-cell numbers in the peripheral blood and bone marrow
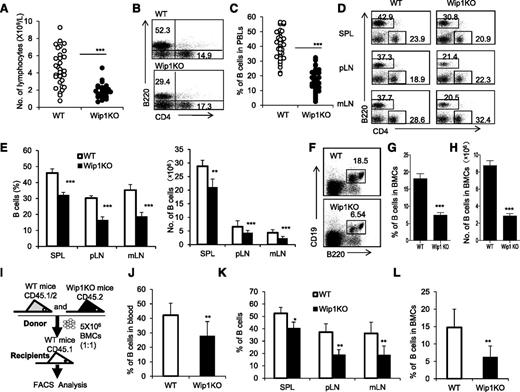
To investigate the involvement of Wip1 in B-cell development, we used Wip1KO mice and analyzed the number of B cells in the peripheral blood, spleens, lymph nodes (LNs), and bone marrow of 6- to 8-week-old Wip1KO mice and their WT littermates. As shown in Figure 1, a significantly lower number of lymphocytes in the peripheral blood of Wip1KO mice was observed compared with those in the WT mice (P < .001; Figure 1A). FACS analysis showed a significant proportional reduction of B220+ B cells in the peripheral blood of Wip1KO mice compared with those in the WT mice (P < .001; Figure 1B-C). Consistent with the alteration of B cells in the peripheral blood, the percentage and number of B220+ B cells in the spleens, peripheral LNs (pLNs), and mesentery LNs (mLNs) in Wip1KO mice were significantly decreased compared with those in the WT mice (P < .001; Figure 1D-E). The percentage and number of T cells in the spleens and pLNs in Wip1KO mice were somewhat increased or unchanged compared with those in the WT mice (supplemental Figure 1, available on the Blood Web site). Importantly, the percentage and number of B220+CD19+ B cells in the bone marrow of Wip1KO mice were also significantly less than those in WT mice (P < .01; Figure 1F-H). Thus, Wip1 deficiency caused a significant decrease in B cells in the peripheral immune tissues and bone marrow in mice.
Wip1KO mice exhibit a reduction of B cells in the periphery immune tissues and bone marrow due to a cell-intrinsic defect. (A) Total number of lymphocytes in the peripheral blood of 6- to 8-week-old WT and Wip1KO mice was determined using a blood cell counter. Data are expressed as means ± SD (n ≥ 25). (B) Representative FACS plots of B cells and CD4+ T cells in the peripheral blood of WT and Wip1KO mice. (C) The percentage of B cells in the peripheral blood of WT and Wip1KO mice (n ≥ 25). (D) Representative FACS plots of B cells and CD4+ T cells in the spleen, pLNs, and mLNs of 6- to 8-week-old WT and Wip1KO mice. (E) The bar graphs show the percentage and number of B cells in the spleen, pLNs, and mLNs of WT and Wip1KO mice (n = 8). (F) Representative FACS plots of B cells in the bone marrow of 6- to 8-week-old WT and Wip1KO mice. (G) The percentage of B cells in the bone marrow was significantly lower in Wip1KO mice than in WT mice. (H) The number of B cells in the bone marrow was significantly lower in Wip1KO mice than in WT mice (n ≥ 20). (I) The scheme of the experimental design for bone marrow transplantations. (J) The percentage of WT and Wip1KO donor-derived B cells in the peripheral blood of recipient mice 12 weeks after transplantation (n = 3-5). (K) The percentage of WT and Wip1KO donor-derived B cells in the donor-derived cells in the spleens, pLNs, and mLNs of mixed-chimera recipients 12 weeks after transplantation is shown (n = 3-5). (L) The percentage of WT and Wip1KO donor-derived B cells in the bone marrow of mixed-chimera recipients 12 weeks after transplantation is summarized (n = 3-5). Data are expressed as means ± SD. *P < .05, **P < .01, and ***P < .001 compared with WT mice. PBLs, peripheral blood lymphocytes; SPL, spleen.
Wip1KO mice exhibit a reduction of B cells in the periphery immune tissues and bone marrow due to a cell-intrinsic defect. (A) Total number of lymphocytes in the peripheral blood of 6- to 8-week-old WT and Wip1KO mice was determined using a blood cell counter. Data are expressed as means ± SD (n ≥ 25). (B) Representative FACS plots of B cells and CD4+ T cells in the peripheral blood of WT and Wip1KO mice. (C) The percentage of B cells in the peripheral blood of WT and Wip1KO mice (n ≥ 25). (D) Representative FACS plots of B cells and CD4+ T cells in the spleen, pLNs, and mLNs of 6- to 8-week-old WT and Wip1KO mice. (E) The bar graphs show the percentage and number of B cells in the spleen, pLNs, and mLNs of WT and Wip1KO mice (n = 8). (F) Representative FACS plots of B cells in the bone marrow of 6- to 8-week-old WT and Wip1KO mice. (G) The percentage of B cells in the bone marrow was significantly lower in Wip1KO mice than in WT mice. (H) The number of B cells in the bone marrow was significantly lower in Wip1KO mice than in WT mice (n ≥ 20). (I) The scheme of the experimental design for bone marrow transplantations. (J) The percentage of WT and Wip1KO donor-derived B cells in the peripheral blood of recipient mice 12 weeks after transplantation (n = 3-5). (K) The percentage of WT and Wip1KO donor-derived B cells in the donor-derived cells in the spleens, pLNs, and mLNs of mixed-chimera recipients 12 weeks after transplantation is shown (n = 3-5). (L) The percentage of WT and Wip1KO donor-derived B cells in the bone marrow of mixed-chimera recipients 12 weeks after transplantation is summarized (n = 3-5). Data are expressed as means ± SD. *P < .05, **P < .01, and ***P < .001 compared with WT mice. PBLs, peripheral blood lymphocytes; SPL, spleen.
Wip1 intrinsically controls B-cell development
Wip1 controls granulocyte development and inflammatory function in mice.16-18 Neutrophil activation in the bone marrow greatly alters B-cell development in human and mouse systemic lupus erythematosus.27 Thus, we wanted to know whether Wip1 affects the B-cell population intrinsically or the impaired B-cell levels in Wip1KO mice are caused by indirect pathways such as activated neutrophils. This question was addressed with full and mixed bone marrow chimera mouse models. When we adoptively transferred WT or Wip1-deficient BMCs, respectively, into lethally irradiated syngeneic recipients to establish full hematopoietic chimeras (supplemental Figure 2A), a significantly lower percentage of B220+ B cells and a higher percentage of myeloid cells (CD11b+) were observed in recipients that received Wip1-deficient BMCs compared with those that received WT BMCs (P < .01; supplemental Figure 2B-C). Furthermore, the percentage of B220+CD19+ B cells in the bone marrow was also significantly lower in recipients that received Wip1KO BMCs than in those that received WT BMCs (P < .001; supplemental Figure 2D). Reversely, when lethally irradiated WT or Wip1KO mice received WT BMCs to establish full chimeras (supplemental Figure 2E), Wip1-deficient mice grafted with WT BMCs showed similar levels of B cells in the peripheral blood and bone marrow as WT mice that received WT BMCs (supplemental Figure 2F-H). These data indicate that the deficiency of Wip1 in nonhematopoietic cells failed to impact B-cell development in a cell-extrinsic manner. Additionally, we generated mixed hematopoietic chimeras in which lethally irradiated congenic WT CD45.1+ mice were cotransplanted 5 × 106 CD45.1/CD45.2+ WT BMCs with 5 × 106 CD45.2+ Wip1-deficient BMCs (Figure 1I). In this model, the percentage of Wip1KO B cells in CD45.2+ cells (Wip1KO) was significantly lower than WT B cells in CD45.1/CD45.2+ cells (WT) in all detected immune tissues, including the peripheral blood, spleens, LNs, and bone marrow of mixed chimeras (P < .01; Figure 1J-L). Because the simultaneous presence of WT BMCs and immune cells in the same host failed to rescue the B-cell alteration caused by Wip1 deficiency and normal levels of B cells were detected in WT BMC-grafted Wip1KO mice, we conclude that Wip1 regulates B-cell development in a cell-autonomous manner.
Wip1 deficiency impairs early B-cell development in the bone marrow
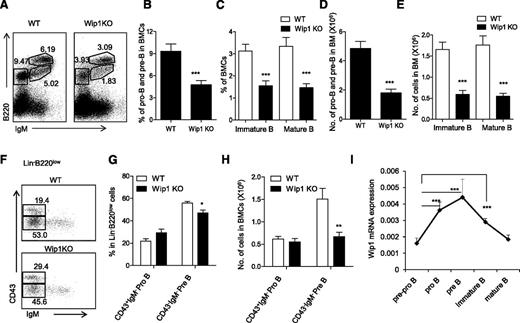
Bone marrow common lymphoid progenitors (CLPs) are lymphoid restricted and can generate B cells and T cells. We have examined the number of CLPs in 6- to 8-week-old mice and excluded the possibility that Wip1 deficiency causes a significant defect of CLPs at least in young mice (supplemental Figure 3A-B). We thus focused our efforts to further explore the role of Wip1 in the development of B cells from their progenitors and precursors. B-cell development is a highly ordered process by the rearrangement of immunoglobulin heavy-chain and light-chain gene segments, which can be defined in 3 developmental stages. Immunoglobulin gene heavy-chain D to J rearrangement occurs in CD19+B220+CD43+ pro-B cells; V to DJ rearrangement occurs for further progression to B220midCD43−IgM− pre–B cells, followed by light-chain rearrangement and formed IgM expression on the cell surface of B220midCD43−IgM+ immature B cells and, finally, B220highCD43−IgM+ mature B cells.28-30 As shown in Figure 2, the numbers of all 3 of these B-cell fractions, including pro-B and pre–B cells (B220+IgM−), immature B cells (B220midIgM+), and mature B cells (B220highIgM+), were significantly decreased in the bone marrow of Wip1KO mice compared with those in the WT mice, respectively (P < .01; Figure 2A-E). These data indicate that the defect in B-cell development caused by Wip1 deficiency occurred at as early as the pre–B-cell stage. To determine whether even earlier stages, like pro–B cells, were impacted by Wip1 deficiency, we performed multicolor flow cytometry to analyze pro–B cells and pre–B cells in gated Lin−B220low BMCs. The percentage of CD43+IgM− pro–B cells was increased and the percentage of CD43−IgM− pre−B cells was decreased in the gated Lin−B220low BMCs of Wip1KO mice compared with WT mice (P < .05; Figure 2F-G). The total number of CD43+IgM− pro–B cells in Wip1KO mice was identical to that in the WT mice, but the number of CD43−IgM− pre–B cells in Wip1KO mice was significantly decreased compared with WT mice (P < .001; Figure 2H). Therefore, the impairment of B-cell development in Wip1KO mice is mainly due to a severe blockage at the pre–B-cell stage. This conclusion was further supported by the Wip1 expression pattern during B-cell development. Quantitative PCR analysis of purified pre-pro–B (B220+CD43+CD19−), pro-B (B220+CD43+CD19+), pre-B (B220midCD43−IgM−CD19+), immature B (B220midCD43−IgM+CD19+), and mature B (B220hiCD43−IgM+CD19+) cells from the bone marrow of WT mice revealed an increased expression of Wip1 in the pro-B and pre–B cells, implicating a potential role of Wip1 in the early development of B cells in the bone marrow (Figure 2I). The high expression level of Wip1 in sorted pre–B cells among all B-cell developing stages offered a material base for the potential direct effects of Wip1 on pre–B-cell development.
Wip1 deficiency impairs early B-cell development. (A) Representative FACS plots of developing B cells in the bone marrow of WT and Wip1KO mice are shown. The percentage of pro-B and pre–B cells (B) and immature and mature B cells (C) in WT and Wip1KO mice is shown. The number of pro-B and pre–B cells (D) and immature and mature B cells (E) in WT and Wip1KO mice is summarized. (F) Representative FACS plots of pro-B and pre–B cells in the gated Lin−B220low BMCs. (G-H) The percentage and number of pro-B and pre–B cells in the bone marrow of WT and Wip1KO mice are summarized. Data are expressed as means ± SD (n ≥ 30). (I) Wip1 mRNA expression in pre-pro–B, pro-B, pre-B, immature B, and mature B cells of 2-month-old mice was analyzed by real-time PCR. Results are standardized to β-actin and expressed as means ± SD (n = 5). *P < .05, **P < .01, and ***P < .001 compared with WT mice or between the indicated groups.
Wip1 deficiency impairs early B-cell development. (A) Representative FACS plots of developing B cells in the bone marrow of WT and Wip1KO mice are shown. The percentage of pro-B and pre–B cells (B) and immature and mature B cells (C) in WT and Wip1KO mice is shown. The number of pro-B and pre–B cells (D) and immature and mature B cells (E) in WT and Wip1KO mice is summarized. (F) Representative FACS plots of pro-B and pre–B cells in the gated Lin−B220low BMCs. (G-H) The percentage and number of pro-B and pre–B cells in the bone marrow of WT and Wip1KO mice are summarized. Data are expressed as means ± SD (n ≥ 30). (I) Wip1 mRNA expression in pre-pro–B, pro-B, pre-B, immature B, and mature B cells of 2-month-old mice was analyzed by real-time PCR. Results are standardized to β-actin and expressed as means ± SD (n = 5). *P < .05, **P < .01, and ***P < .001 compared with WT mice or between the indicated groups.
p53 deletion, but not p21 deletion, rescues the defect in B-cell development in Wip1KO mice
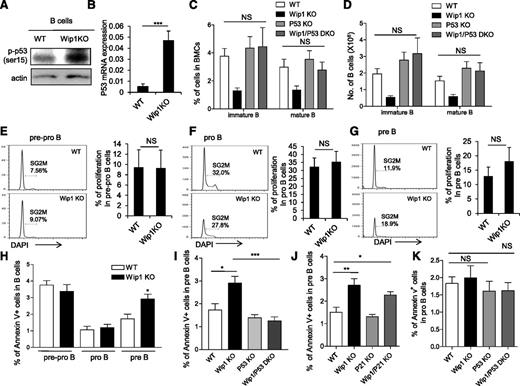
Considering that the regulatory loop between Wip1 and p53 in tumor cells and a sustained activation of p53 has been shown in the thymocytes of Wip1KO mice,10,19-21 we examined the level of p53 in the B220+ B cells from the bone marrow of WT and Wip1KO mice. Interestingly, phosphorylated p53 protein levels were elevated in the total B cells and sorted pre–B cells of Wip1KO mice (Figure 3A and supplemental Figure 4A-B). In addition, the mRNA level of p53 was increased in the pre–B cells of Wip1KO mice as determined by real-time PCR (P < .001; Figure 3B). To genetically prove the hypothesis that activation of the p53 pathway is responsible for the impairment of B-cell development induced by Wip1 deficiency, we generated Wip1/p53 DKO mice. FACS analysis showed that the percentage and number of pro-B, pre-B, immature B, and mature B cells in the bone marrow of Wip1/p53 DKO mice were restored to levels that are comparable to those in p53KO and WT mice (Figure 3C-D and supplemental Figure 5A-B), indicating that the deletion of p53 completely rescued the impairment of B-cell development in the bone marrow of Wip1KO mice. This observation is consistent with previous reports showing the critical role of p53 in early B-cell development and malignant transformation in the bone marrow of p53-deficient mice.31,32 Because the cyclin-dependent kinase inhibitor p21, a downstream element of p53, controls cell-cycle progression from G1 to S phase, we thus also analyzed B-cell development in Wip1/p21 DKO mice. However, similar levels of pro-B, pre-B, immature, and mature B cells were observed in Wip1KO and Wip1/p21 DKO mice (supplemental Figure 5C-F), suggesting that Wip1 deficiency induces a defect in B-cell development through a p21-independent mechanism.
p53 deletion, but not p21 deletion, rescues the B-cell development defect in the bone marrow of Wip1KO mice. (A) Western blot analysis of phospho-p53 (ser 15) expression in sorted B220+ cells from the bone marrow of WT and Wip1KO mice. Western blots are representative of 3 independent experiments. (B) Relative expression of p53 in FACS sorted pre–B cells. Data are expressed as means ± SD (n = 6). (C) The percentage of immature B cells and mature B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53-DKO mice. (D) The number of immature B cells and mature B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53-DKO mice. Data are expressed as means ± SD (n = 7). Representative histograms show the cell-cycle profile of early B-cell precursors, including pre-pro–B cells (E), pro–B cells (F), and pre–B cells (G). The bar graphs show the percentage of pre-pro–B, pro-B, and pre–B cells in SG2M phase. Data are expressed as means ± SD (n = 9). (H) The bar graphs show the percentage of B cells that are Annexin V positive in pre-pro–B, pro-B, and pre–B cells in WT and Wip1KO mice. Data are expressed as means ± SD (n = 8). (I) The bar graphs show the percentage of Annexin V–positive pre–B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53 DKO mice (n = 6). (J) The bar graphs show the apoptosis of pre-B cells in the bone marrow of WT, Wip1KO, p21KO, and Wip1/p21 DKO mice. (K) The percentage of Annexin V–positive pro–B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53 DKO mice. No significant difference was observed when comparing apoptosis in pro–B cells in Wip1KO, p53KO, and Wip1/p53-DKO mice with that in WT mice. Data are expressed as means ± SD (n = 5). *P < .05, **P < .01, and ***P < .001 compared with WT mice or between the indicated groups. NS, not significant.
p53 deletion, but not p21 deletion, rescues the B-cell development defect in the bone marrow of Wip1KO mice. (A) Western blot analysis of phospho-p53 (ser 15) expression in sorted B220+ cells from the bone marrow of WT and Wip1KO mice. Western blots are representative of 3 independent experiments. (B) Relative expression of p53 in FACS sorted pre–B cells. Data are expressed as means ± SD (n = 6). (C) The percentage of immature B cells and mature B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53-DKO mice. (D) The number of immature B cells and mature B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53-DKO mice. Data are expressed as means ± SD (n = 7). Representative histograms show the cell-cycle profile of early B-cell precursors, including pre-pro–B cells (E), pro–B cells (F), and pre–B cells (G). The bar graphs show the percentage of pre-pro–B, pro-B, and pre–B cells in SG2M phase. Data are expressed as means ± SD (n = 9). (H) The bar graphs show the percentage of B cells that are Annexin V positive in pre-pro–B, pro-B, and pre–B cells in WT and Wip1KO mice. Data are expressed as means ± SD (n = 8). (I) The bar graphs show the percentage of Annexin V–positive pre–B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53 DKO mice (n = 6). (J) The bar graphs show the apoptosis of pre-B cells in the bone marrow of WT, Wip1KO, p21KO, and Wip1/p21 DKO mice. (K) The percentage of Annexin V–positive pro–B cells in the bone marrow of WT, Wip1KO, p53KO, and Wip1/p53 DKO mice. No significant difference was observed when comparing apoptosis in pro–B cells in Wip1KO, p53KO, and Wip1/p53-DKO mice with that in WT mice. Data are expressed as means ± SD (n = 5). *P < .05, **P < .01, and ***P < .001 compared with WT mice or between the indicated groups. NS, not significant.
Given the fact that Wip1KO mice exhibit a dramatic reduction of pre–B cells, immature B cells, and mature B cells, which can be rescued by p53 deletion, we examined the cell-cycle profile by Ki67/DAPI staining and apoptosis by Annexin V/DAPI staining at various stages of B-cell development. Cell-cycle analyses did not detect a significant difference in the pre-pro–B, pro-B, and pre–B-cell compartments between WT and Wip1KO mice (Figure 3E-G and supplemental Figure 5G-I). Interestingly, we detected a significantly higher percentage of Annexin V–positive cells in the pre–B-cell compartment of Wip1KO mice compared with that in WT mice, indicating that pre–B cells are more prone to undergo apoptosis in the context of Wip1 deficiency (Figure 3H). However, the percentages of apoptotic pre-pro–B and pro–B cells were comparable between WT mice and Wip1KO mice in steady state (Figure 3H). Next, we examined apoptosis in pre–B cells of Wip1/p53 DKO mice and Wip1/p21 DKO mice. The results showed that the increased apoptosis in Wip1KO pre–B cells was significantly rescued in Wip1/p53 DKO mice, but not in Wip1/p21 DKO mice (Figure 3I-J). On the other hand, we failed to detect a significant difference when comparing apoptosis in pro–B cells in Wip1KO and p53KO Wip1/p53 KO mice with that in WT mice (Figure 3K). These data collectively indicate that Wip1 deficiency activates a p53-dependent apoptotic process limiting the survival of pre–B cells.
Impaired B-cell development in Wip1KO mice is exacerbated by aging
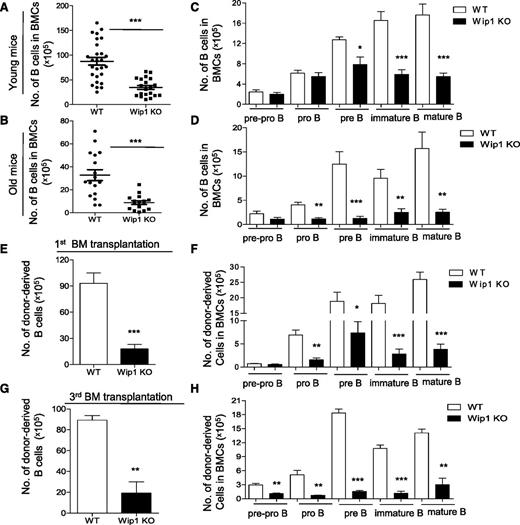
One of the aging characteristics of the hematopoietic system is reduced lymphopoiesis in both humans and mice.33-35 Therefore, we examined B-cell development in 20- to-24-month-old WT and Wip1KO mice. Similar to the impairment in young Wip1KO mice, FACS analysis revealed a significant reduction in total B-cell numbers in the bone marrow of old Wip1KO mice compared with age-matched WT mice (Figure 4A-B), and the defect was also cell intrinsic (supplemental Figure 6A). However, the impairment of the pre–B-cell compartment in the bone marrow of Wip1KO mice was further exacerbated by aging (Figure 4C-D). Annexin V staining revealed that the increase in apoptosis induced by Wip1 deficiency becomes more dramatic in old Wip1KO mice compared with young Wip1KO mice (supplemental Figure 6B).
Impaired B-cell development in Wip1KO mice is exacerbated by aging and serial transplantation. The number of total B cells in the bone marrow of WT and Wip1KO mice at a young (2 months, A) and old (20-24 months, B) age is summarized. The number of the developing B cells, including pre-pro–B, pro-B, pre-B, immature B, and mature B cells in the bone marrow of WT and Wip1KO mice at a young (C) and old (D) age is shown. Data are expressed as means ± SD (n ≥ 12). (E-H) The bar graphs show the number of B, pre-pro–B, pro-B, pre-B, immature B, and mature B cells in donor-derived BMCs 20 weeks after the first transplantation (E-F) and after the third transplantation (G-H). Data are expressed as means ± SD (n = 8). *P < .05, **P < .01, and ***P < .001 compared with WT mice or between the indicated groups. BM, bone marrow.
Impaired B-cell development in Wip1KO mice is exacerbated by aging and serial transplantation. The number of total B cells in the bone marrow of WT and Wip1KO mice at a young (2 months, A) and old (20-24 months, B) age is summarized. The number of the developing B cells, including pre-pro–B, pro-B, pre-B, immature B, and mature B cells in the bone marrow of WT and Wip1KO mice at a young (C) and old (D) age is shown. Data are expressed as means ± SD (n ≥ 12). (E-H) The bar graphs show the number of B, pre-pro–B, pro-B, pre-B, immature B, and mature B cells in donor-derived BMCs 20 weeks after the first transplantation (E-F) and after the third transplantation (G-H). Data are expressed as means ± SD (n = 8). *P < .05, **P < .01, and ***P < .001 compared with WT mice or between the indicated groups. BM, bone marrow.
To further investigate whether Wip1 deficiency accelerated the B-cell decline in replicative aging, we used a serial transplantation mouse model with WT and Wip1KO mouse BMCs. We analyzed the frequency of the B-cell component, including B-cell progenitors and precursors, in these mixed bone marrow chimera mice. The reduction of B cells, including mature, immature, pre–B cells, and pro–B cells, in Wip1KO donor-derived BMCs became more dramatic in the primary recipient mice (Figure 4E-F). In the tertiary recipient mice, the defect of the pre–B-cell compartment in Wip1KO donor-derived BM cells became more severe compared with the first bone marrow–transplanted mice (Figure 4G-H). Further analysis of the donor-derived BMCs in recipient mice showed that transplantation exacerbates Wip1-deficiency–induced apoptosis in pre–B cells (supplemental Figure 6C). These data indicate that both physiological aging and experimentally forced replicative aging exacerbate the impairment of the pre–B-cell compartment in the context of Wip1 deficiency.
In addition, the data also showed that aging and transplantation produced extra defects in the pro–B-cell compartment (Figure 4D,F,H) and in the pre-pro–B-cell compartment after serial transplantation (Figure 4H). This appears to contradict the earlier conclusion that numbers of these populations are unaffected. To further investigate whether this phenotype could be due to a newly involved defect in early lymphoid progenitors during aging and after transplantation, we examined the CLPs in these mice. The data revealed a significant reduction of CLPs both in the old Wip1KO mice and in the Wip1KO donor-derived BMCs after transplantation (supplemental Figure 6D-G). Next, we investigated whether p53 deficiency could restore the reduction of CLP numbers in the transplantation setting. The number of Wip1/p53 DKO donor-derived CLPs was significantly less than those of p53KO donor-derived CLPs in the recipient mice, indicating a p53-independent mechanism impairing CLP maintenance (supplemental Figure 6H). Unfortunately, we were not able to investigate whether p53 deficiency could restore the age-associated decline in CLPs in the context of Wip1 deficiency, as the Wip1/p53 DKO mice died within 1 year of age. However, significantly increased Wip1 expression in developing B cells in p53KO mice was observed (supplemental Figure 7C), indicating the regulatory loop between p53 and Wip1 molecules. Together, our study shows that replicative aging exacerbates a Wip1-deficiency–induced B-cell defect through not only a p53-dependent defect dominantly affecting pre–B cells, but also a p53-independent defect dominantly effecting CLPs in aged mice. However, the number of splenic T cells was similar in old Wip1KO mice and age-matched WT mice (supplemental Figure 7A-B), as reported by Schito et al.19
Discussion
Wip1 has been shown to inhibit DNA damage checkpoints, DNA repair, senescence, apoptosis, and the production of inflammatory cytokines in various contexts.36-38 The effects of Wip1 deletion in T-cell maturation and neutrophil maturation have been well studied in mice. The impairment in T-cell maturation is due to a p53-dependent cell-cycle arrest,19 whereas the hypermaturation of neutrophils is mediated by p38 mitogen-activated protein kinase/signal transducer and activator of transcription 1–dependent pathways.16 In addition, overexpression of Wip1 has been observed in several types of human cancers, including B lymphomas.21 However, the role of Wip1 in B-cell development is still unknown. Here, we report a novel function of Wip1 in regulating early B-cell development in the bone marrow.
Previous studies showed a decreasing tendency in the percentage of CD19+ cells in the spleen of Wip1KO mice,22 although the differences failed to achieve statistical significance, probably due to a high standard variation and limited mouse numbers in the Wip1KO group. In addition, the discrepancy between these studies could be caused by the difference in genetic backgrounds and the age of the mice that were analyzed. Regarding peripheral T cells, our studies showed that an increase in splenic T cells was observed in young Wip1KO mice, but T-cell numbers were similar in old Wip1KO mice and WT mice (supplemental Figures 1A-B and 7A-B), as reported by Schito et al.19 In addition, another study by Choi et al showed a higher frequency of CD4 T cells but a lower frequency of CD8 T cells in the spleen of Wip1KO mice.22 Our data showed no significant difference in the ratio of CD4/CD8 T cells in the spleen of Wip1KO and WT mice at either a young or an old age. This discrepancy could be due to the genetic backgrounds and age of the mice that were analyzed.
Our studies with full or mixed bone marrow chimera mice showed that Wip1 is an intrinsic factor maintaining B-cell development. In mixed-chimera mice, Wip1-deficient BMCs differentiated into B cells in a lower efficiency compared with the WT BMCs. Impaired B-cell development in Wip1-deficient mice is due to an enhanced apoptosis mediated by p53, especially at the pre–B-cell stage, as supported by the following evidence: (1) Wip1-deficient B cells express high levels of p53 as detected by real-time PCR and western blots; (2) deletion of p53 remarkably secured the poor B-cell development caused by Wip1 deficiency; and (3) Wip1 deficiency caused more cell death in pre–B cells, whereas simultaneous p53 deletion significantly reversed it. On the other hand, the cyclin-dependent kinase inhibitor p21, as a downstream element of p53, controls cell-cycle progression from G1 to S phase. However, p21 deletion failed to rescue B-cell impairment in Wip1-deficient mice, which reinforced the notion that Wip1 deficiency resulted in B-cell defects through p53-mediated apoptosis.
Antigen-independent B-cell development in bone marrow is subject to apoptosis and macrophage-mediated cell elimination.39 p53 has been reported to impact B-cell development by reducing apoptosis.40 In line with this, our studies showed that suppression of p53-dependent apoptosis is critical for early B-cell development. Interestingly, a previous study reported that p53 deficiency mainly affected pro–B cells, whereas our studies showed that pre–B cells were predominantly affected by sustained p53 activation in the context of Wip1 deficiency. In fact, we also observed a tendency of decreasing apoptosis in pro–B cells in p53KO mice, although it was not statistically significant. There are a few differences between the 2 studies: (1) the previous study used 129/Sv mice, whereas we used C57Bl/6 mice; (2) the previous study used B220+μ− to define pro–B cells, whereas we used Lin−B220lowCD43+IgM−CD19+ to define pro–B cells; and (3) the previous study used the hypodiploid region of DNA content profiles as apoptotic cells, whereas we used Annexin V+ and DAPI− to measure early apoptotic cells. Such differences may explain the discrepancy in the results obtained in different studies.
Wip1 has been proposed as a key mediator in p53 oscillation.41 In line with previous reports,40,42 our studies reinforced the notion that, during B-cell development, DNA recombination events induce DNA damage responses resulting in p53 activation and apoptosis. As a negative feedback loop, p53 activation leads to upregulation of Wip1, which in turn dampens p53 protein levels. Indeed, we have observed sustained p53 activation in Wip1-deficient B cells and higher Wip1 expression in p53KO B-cell populations. The impact of p53-dependent apoptosis on B-cell development in Wip1KO mice is more obvious at the pre–B-cell stage, which could be due to the possibility that a Wip1-p53 negative feedback loop becomes more necessary at this stage. Moreover, even though the increase in apoptosis in Wip1KO pre–B cells was only 1.5-fold, it resulted in a twofold decrease in cell numbers. There are 2 possible explanations: (1) the in vivo incidence of apoptosis was often underestimated, as the early apoptotic cells are quickly cleared by macrophages; and (2) the apoptosis assay was merely reflecting a snapshot, and the effect accumulated over time. Taken together, our data indicate that Wip1 plays an important role in maintaining the homeostatic level of p53 to support B-cell development, especially at the pre–B-cell stage.
The immune system undergoes dramatic age-related changes resulting in a decline in responses to vaccination or infection, diminished protective immunity, and increased disease morbidity. Previous studies have consistently shown that old WT mice exhibit a decreased frequency of early B-cell precursors and reduced production of naive B cells. The molecular mechanism of B-cell aging is still not clear. Accumulating evidence suggests that p53 and p16 are involved in promoting cellular senescence or apoptosis. Wip1 functions as a negative feedback mechanism to dampen p53 activation. Therefore, it is of interest to understand the role of Wip1 in the p53-dependent regulation of B-cell development during aging. In our study, Wip1 deficiency resulted in a p53-dependent impairment in B-cell development, especially at the pre–B-cell stage, which was exacerbated during aging and serial transplantation. In addition, physiological aging and experimental enforced replicative aging resulted in an extra defect involving a decline of CLPs. Consistent with this, accumulating results suggest that Wip1 is involved in several aging-related physiological and pathological processes.11,12,43,44 In addition to the previous findings that suggest a role of Wip1 deficiency in cell-cycle arrest or cellular senescence in tissue aging, our study has demonstrated a novel function of Wip1 in preventing p53-mediated cell death in early B-cell precursors during aging and serial transplantation.
In summary, our data show that Wip1 deficiency results in a B-cell development defect through enhanced p53-dependent apoptosis in early B-cell precursors. These findings shed light on the general interests of the molecular mechanisms that regulate B-cell development during aging and replication stresses and therefore have important implications for clinical applications in immune dysfunction and stem cell therapies.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Chenming Sun and Lina Sun for their kind review of the manuscript. The authors are grateful to Dr L. A. Donehower from Baylor College of Medicine for providing Wip1-knockout mice.
This work is supported by the National Basic Research Program of China (2011CB964802, 2011CB710903, and 2012CB911203), the National Natural Science Foundation of China (81130074, 81420108017, 81222003, C81130055, C81072396, and 31300724), and the Chinese Academy of Sciences/State Administration of Foreign Experts Affairs (CAS/SAFEA) International Partnership Program for Creative Research Teams.
Authorship
Contribution: W.Y., X.H., and Z.C. designed and carried out the experiments and analyzed data; W.Y., Z.C., and Y.T. bred mice and performed PCR assays; W.Y., L.L., and H.C. performed flow cytometry assays; Y.C. performed real-time PCR assays; F.Y. typed the genetically modified mice; L.Z. provided animal models and revised the manuscript; K.L.R. provided animal models and comments; and Z.Z., Y.Z., and Z.J. designed experiments, analyzed data, wrote the manuscript, and provided overall supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhenyu Ju, Institute of Aging Research, Hangzhou Normal University School of Medicine, Wen Yi Xi Rd 1378, Hangzhou, Zhejiang 311121, China; e-mail: zhenyuju@163.com; Yong Zhao, Transplantation Biology Research Division, State Key Laboratory of Biomembrane and Membrane Biotechnology, Institute of Zoology, Chinese Academy of Sciences, Beichen Western Rd 1-5, Chaoyang District, Beijing 100101, China; e-mail: zhaoy@ioz.ac.cn; and Zhixin Zhang, Department of Pediatrics, West China Second University Hospital, State Key Laboratory of Biotherapy, Ministry of Education Key Laboratory of Birth Defects, Sichuan University, Chengdu 610041, China; e-mail: zhangzhixin@scu.edu.cn.
References
Author notes
W.Y., X.H., and Z.C. contributed equally to this study.