Key Points
VAMP-7 functions in platelet granule exocytosis and spreading.
VAMP-7 associates with VARP and Arp2/3, thereby linking granule exocytosis and actin reorganization.
Abstract
Platelet activation results in profound morphologic changes accompanied by release of granule contents. Recent evidence indicates that fusion of granules with the plasma membrane during activation provides auxiliary membrane to cover growing actin structures. Yet little is known about how membrane fusion is coupled with actin reorganization. Vesicle-associated membrane protein (VAMP)-7 is found on platelet vesicles and possesses an N-terminal longin domain capable of linking exocytosis to cytoskeletal remodeling. We have evaluated platelets from VAMP-7−/− mice to determine whether this VAMP isoform contributes to granule release and platelet spreading. VAMP-7−/− platelets demonstrated a partial defect in dense granule exocytosis and impaired aggregation. α Granule exocytosis from VAMP-7−/− platelets was diminished both in vitro and in vivo during thrombus formation. Consistent with a role of VAMP-7 in cytoskeletal remodeling, spreading on matrices was decreased in VAMP-7−/− platelets compared to wild-type controls. Immunoprecipitation of VAMP-7 revealed an association with VPS9-domain ankyrin repeat protein (VARP), an adaptor protein that interacts with both membrane-bound and cytoskeleton proteins and with Arp2/3. VAMP-7, VARP, and Arp2/3 localized to the platelet periphery during spreading. These studies demonstrate that VAMP-7 participates in both platelet granule secretion and spreading and suggest a mechanism whereby VAMP-7 links granule exocytosis with actin reorganization.
Introduction
Regulated release of cargo from granules is an essential platelet function that contributes not only to hemostasis and thrombosis but also to inflammation, angiogenesis, atherosclerosis, malignancy, response to invading microbes, and wound healing.1 The importance of dense granule release in hemostasis is evidenced by the bleeding diathesis in patients with Hermansky-Pudlak syndrome, who have a platelet dense granule deficiency.2,3 Patients with gray platelet syndrome, characterized by a platelet α granule deficiency owing to mutations in Nbeal2,4-6 also have a bleeding tendency.7 The role of platelet α granules in other physiologic and pathophysiologic functions is now beginning to be understood. For example, Nbeal2−/− mice experience delayed wound healing after full-thickness dermal injury and show marked protection from cancer metastasis.8,9
Several components of the molecular machinery responsible for platelet granule exocytosis have been identified. In particular, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and their chaperones (such as N-ethylmaleimide–sensitive factor, Munc13-4, Munc18-2, Slp4, and STXBP5) have been shown to regulate membrane fusion required for granule exocytosis.10-16 One family of SNAREs, the target SNAREs, associate primarily with target membranes such as the open canalicular system and plasma membrane.17 In platelets, target SNARE function appears to be mediated primarily by soluble n-ethylmaleimide-sensitive-factor attachment protein (SNAP)-23 and syntaxin-11, with a potential contribution from syntaxin-8.18-22 Vesicle-associated membrane proteins (VAMPs) belong to the vesicle (v)-SNARE family and reside on the cytoplasmic surface of platelet granules. Platelets contain several VAMP isoforms, including VAMP-2, VAMP-3, VAMP-7, and VAMP-8, which play a role in granule trafficking and secretion.23-27 VAMP-8 is the dominant v-SNARE in platelets and regulates both α and dense granule release.24 VAMP-2 and VAMP-3 appear to have a compensatory role in platelet granule exocytosis.
VAMP-7 (also known as tetanus neurotoxin–insensitive VAMP) is an ∼25-kD protein that is relatively abundant in platelets (copy number 3766 ± 696).25 VAMP-7 is unique among platelet VAMP isoforms in that it contains an N-terminal longin domain upstream of its SNARE motif.28,29 In addition to its role in cargo release, VAMP-7 mediates fusion events required for membrane remodeling functions in a variety of cells. It participates in neurite outgrowth,30-32 plasma membrane remodeling associated with phagocytotic cup formation in macrophages,33,34 membrane resealing,35,36 lysosome secretion during cell migration,37 vesicular transport to the apical membrane in epithelial cells,38 and release of autophagic vesicles.39 In platelets, α granules expressing VAMP-7 move to the periphery of the platelets during spreading.40 However, the role of VAMP-7 in platelet function is unknown.
Concurrent with exocytosis, platelets undergo a dramatic change in shape driven by reorganization of the actin cytoskeleton and characterized by the appearance of masses of polymerized insoluble cytoplasmic actin filaments and extension of pseudopodia. In many nucleated cells, exocytosis of vesicles is controlled in part by the cytoskeleton, with actin dynamics participating in a terminal step in membrane fusion.41,42 We have previously shown that the actin cytoskeleton participates in platelet granule exocytosis.43 Conversely, granules contribute to actin-mediated platelet spreading, as evidenced by the fact that spreading is impaired in platelets from patients with gray platelet syndrome or in mouse platelets that lack Munc13-4, a chaperone protein required for granule exocytosis.40 Yet the molecular underpinnings of the interactions between granule exocytosis and platelet-shape change remain poorly understood.
We now evaluate the role of VAMP-7 in platelet function and identify its binding partners. We find that VAMP-7−/− platelets demonstrate defects in both exocytosis and spreading. Platelet VAMP-7 associates with proteins that interact with the actin cytoskeleton, including VPS9-domain ankyrin repeat protein (VARP) and Arp2/3, and appears to link granule exocytosis and actin reorganization.
Methods
Antibodies and reagents
Antibodies directed against syntaxin-11, SNAP-23 (rabbit polyclonal), and VAMP-7 (mouse monoclonal and rabbit polyclonal) were purchased from Synaptic Systems. Antibodies directed against VARP and syntaxin-4 (rabbit polyclonal) were from Abcam. Anti-Arp2/3 complex, clone 13C9 mouse monoclonal antibody, was obtained from Millipore. Anti–platelet factor 4 (PF4) antibody was purchased from R&D systems. Phycoerythrin-conjugated anti-human/mouse CD62P (P-selectin) antibody was purchased from eBioscience. Anti-phosphotyrosine and anti-phosphoserine/phosphothreonine antibodies were from Abcam. Phycoerythrin-conjugated Jon/A antibody, directed at the activated form of mouse αIIbβ3, was from EMFRET Analytics. Alexa Fluor–conjugated secondary antibodies were purchased from Life Technologies. Thrombin receptor–activating peptide (SFLLRN), the protease-activated receptor 4 (PAR4) agonist peptide AYPGKF, and adenosine 5′-diphosphate (ADP) were from Sigma-Aldrich. Collagen and CHRONO-LUME were from Chrono-log. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich.
Animals
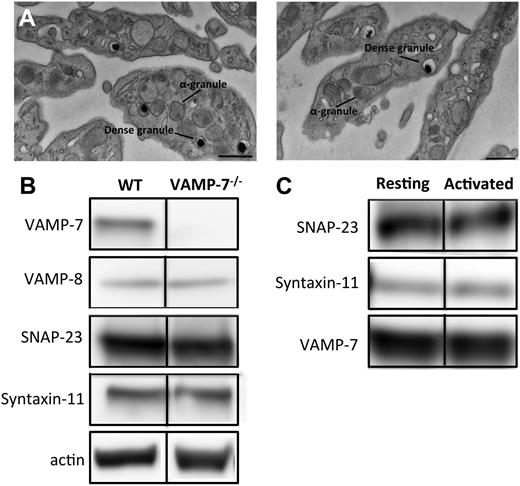
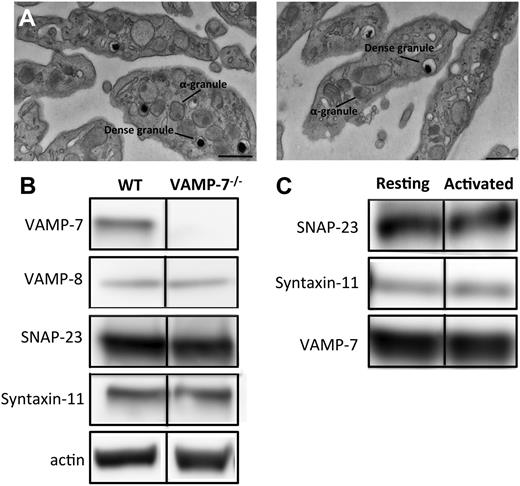
The VAMP7 knockout mouse line was established at the Institut Clinique de la Souris as previously described.44 Briefly, loxP sequences were inserted at the borders of exon 3 of VAMP7 to create a VAMP7 floxed allele. VAMP7flox/flox mice were then crossed with a deleter line expressing Cre recombinase, with the resultant deletion of exon 3. The CMV-Cre::Vamp7flox/flox strain was subsequently crossed with C57BL/6 wild-type animals to eliminate the CMV-Cre transgene.44 Platelets from VAMP-7−/− mice lacked VAMP-7 but were morphologically normal (Figure 1). Male mice used for platelet-function studies or the cremaster injury model were 7 to 14 weeks old and weighed 20 to 28 g.
Characterization of VAMP-7–null platelets. (A) Transmission electron microscopy of platelets from wild-type (left) and VAMP-7−/− mice (right). Scale bars represent 0.5 μm. (B) Immunoblot analysis of lysates derived from wild-type and VAMP-7−/− platelets. (C) Immunoprecipitation of VAMP-7 from resting human platelets (Resting) and platelets stimulated with 5 μM SFLLRN (Activated). SNAP-23, syntaxin-11, and VAMP-7 were subsequently identified in immunoprecipitates using immunoblot analysis. WT, wild-type.
Characterization of VAMP-7–null platelets. (A) Transmission electron microscopy of platelets from wild-type (left) and VAMP-7−/− mice (right). Scale bars represent 0.5 μm. (B) Immunoblot analysis of lysates derived from wild-type and VAMP-7−/− platelets. (C) Immunoprecipitation of VAMP-7 from resting human platelets (Resting) and platelets stimulated with 5 μM SFLLRN (Activated). SNAP-23, syntaxin-11, and VAMP-7 were subsequently identified in immunoprecipitates using immunoblot analysis. WT, wild-type.
Platelet isolation
Platelet spreading assay
Washed platelets were allowed to spread on freshly prepared poly-l-lysine, fibrinogen, or collagen-coated glass coverslips as previously described.40 Briefly, 4 × 106 platelets per 400 μL of platelet suspension was added to the glass coverslip placed within 24-well cell culture plates. Platelets were allowed to adhere/spread for 0, 5, 10, 15, and 30 minutes, followed by fixation using 4% paraformaldehyde in phosphate-buffered saline (PBS). Fixed platelets were washed with PBS and stored in immunofluorescence blocking buffer (1% bovine serum albumin, 10% goat serum, in 1X PBS) at 4°C until staining. Visualization of actin structures was completed using Alexa Fluor 568 Phalloidin (Life Technologies) as directed by the manufacturer. After staining, coverslips were washed and mounted onto glass slides with Aqua-Poly/Mount (Polysciences).
For visualization of actin structures, fluorescent microscopy was performed using an Olympus BX62 microscope (Olympus America) equipped with a 60× (1.42 numerical aperture) Plan Apo oil-immersion objective lens and captured with an ORCA-ER cooled charge-coupled device camera (Hamamatsu). Image acquisitions were controlled by SlideBook 6 (Intelligent Imaging Innovations). All images were exported as tagged image file format files. Using ImageJ software, the surface area and perimeter of platelets were determined. Recorded measurements were analyzed with Prism software.
Immunoprecipitation
Dynabeads (Life Technologies) were used for the immunoprecipitation of VAMP-7 and VARP, and the manufacturer’s protocol was followed with minor changes. Briefly, magnetic beads were incubated with 15 μg of antibody in PBS with 0.02% Tween at room temperature for 30 minutes. Antibody-coated beads were incubated with platelet lysates in radioimmunoprecipitation assay lysis buffer supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific) overnight. Beads were separated by magnetic force and washed 4 times with 0.05% Tween containing PBS buffer (sodium chloride concentration was adjusted to 250 mM to reduce the background). Protein elution from the beads was achieved by suspending beads in Laemmli buffer (Bio-Rad). Coimmunoprecipitated proteins were subsequently analyzed by mass spectrometry and immunoblot analysis.
Immunoblot analysis
Samples were diluted in sample buffer at 95°C for 5 minutes. Proteins were then separated by sodium dodecyl sulfate−polyacrylamide gel electrophoresis. Immunoblotting was performed using Cy2- or Cy5-labeled secondary antibodies (Jackson ImmunoResearch) and visualized using an ImageQuant LAS 4000 (General Electric) for fluorescence detection.
Electron microscopy
Platelet samples for transmission electron microscopy were prepared by fixation of platelets with fixative (2.5% paraformaldehyde, 5% glutaraldehyde, 0.06% picric acid in 0.2 M cacodylate buffer) for 30 minutes at room temperature. After fixation, the platelet pellet was exposed to 1% osmic acid at 4°C for 1 hour. The platelet pellets were then dehydrated slowly in a graded series of alcohol and embedded in the EPON resin. Thin sections were obtained with a diamond knife. Uranyl acetate was used for contrast enhancement.
Aggregation and dense granule secretion
Platelet aggregation and dense granule secretion were measured simultaneously by using a CHRONO-LUME aggregometer as previously described.25
Ca2+ flux measurements
Flow cytometry
Flow cytometry was used to monitor P-selectin expression and to measure surface integrin αIIb levels and activation of integrin αIIbβ3. After exposure of platelets to the indicated concentrations of PAR4 agonist, 5 μL of fluorescent-conjugated antibodies were added to the 25 μL of the platelet suspension (2 × 107 platelets per milliliter) and incubated for 20 minutes at room temperature.48,49 Fluorescence and forward scatter measurements were performed using a Gallios Flow Cytometer (Beckman Coulter). The data were analyzed using Kaluza flow analysis software.
Enzyme-linked immunosorbent assay
PF4 secretion was monitored after treating platelets with PAR4 agonist using a PF4 enzyme-linked immunosorbent assay as described by the manufacturer (R&D Systems).
Laser-induced thrombus formation
The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved all animal care and experimental procedures. Injury to a cremaster arteriolar (30-50 μm diameter) vessel wall was induced with a MicroPoint Laser System (Photonics Instruments).45 Data acquisition was initiated both before and after a single laser pulse for each injury. The microscope system was controlled, and images were analyzed using SlideBook (Intelligent Imaging Innovations) as previously described.45 The data from 23 to 28 thrombi were used to determine the median value of the integrated fluorescence intensity to account for the variability of thrombus formation at any given set of experimental conditions.
Results
Morphologic comparison of wild-type and VAMP-7−/− platelets
VAMP-7–null mice were generated and bred as previously described.44 Loss of VAMP-7 did not result in any overt developmental or neurologic deficits, although decreased brain weight and increased anxiety in behavioral studies have been reported.44 Complete blood counts were performed to identify any gross abnormalities in hematopoiesis. No differences in leukocyte, erythrocyte, and platelet counts between VAMP-7−/− mice and controls were statistically significant (see the supplemental table, available on the Blood Web site). Similarly, no significant differences in mean platelet volume were observed. Evaluation of platelets by transmission electron microscopy demonstrated normal platelet morphology and did not indicate any obvious differences in platelet shape or organelle number (Figure 1A; supplemental Figure 1). Dense granules and α granules in VAMP-7−/− platelets were intact and similar in size and morphology to controls. Immunoblot analysis of lysates from wild-type and VAMP-7−/− platelets demonstrated that VAMP-7−/− mice have platelets with a normal complement of the major platelet SNAREs except for VAMP-7, which is missing from the knockout mice (Figure 1B). Immunoprecipitation of VAMP-7 from human platelets demonstrated that VAMP-7 associates with both SNAP-23 and syntaxin-11 (Figure 1C). In contrast, syntaxin-4 did not coprecipitate with VAMP-7 (not shown).
VAMP-7 functions in platelet aggregation and dense granule secretion
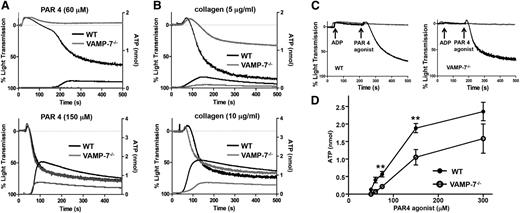
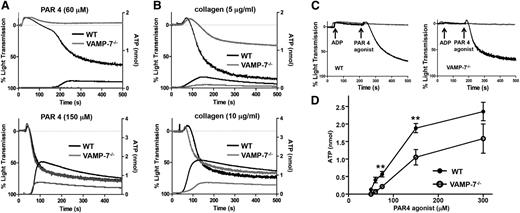
Release of ADP from dense granules provides an autocrine signal that augments agonist-induced platelet aggregation. To evaluate the role of VAMP-7 in platelet aggregation and dense granule release, we simultaneously monitored platelet aggregation by light transmission and dense granule release by luminometry. Platelet aggregation in response to 60 μM AYPGKF, a PAR4 agonist, was markedly reduced in VAMP-7−/− platelets (wild-type, 65.5% ± 10.7% aggregation [n = 6]; VAMP-7−/−, 11.6% ± 5.2% aggregation [n = 5]; P ≤ .002) (Figure 2A). Dense granule release from VAMP-7−/− platelets in response to 60 μM AYPGKF was nearly undetectable (Figure 2A). The defect in aggregation was overcome at 150 μM AYPGKF (wild-type, 81.6% ± 1.4% aggregation [n = 8]; VAMP-7−/−, 79.63% ± 2.0% aggregation [n = 8]; P = .42); however, the defect in dense granule release was not completely reversed at this intermediate dose (Figure 2A). Similarly, platelet aggregation and dense granule release in response to 5 μg/mL collagen were impaired in VAMP-7−/− platelets (wild-type, 64.3% ± 6.4% aggregation [n = 7]; VAMP-7−/−, 26.0% ± 3.8% aggregation [n = 4]; P ≤ .01) (Figure 2B); whereas dense granule release was impaired in VAMP-7−/− platelets exposed to 10 μg/mL collagen, but aggregation was not significantly inhibited (wild-type, 78.5% ± 4.6% aggregation [n = 6]; VAMP-7−/−, 69.8% ± 3.0% aggregation [n = 8]; P = .12). Defective aggregation in VAMP-7−/− platelets was reversed by addition of exogenous ADP, supporting the premise that the aggregation defect was secondary to impaired release of ADP (Figure 2C). The dose response of adenosine triphosphate release to increasing concentrations of AYPGKF confirmed that dense granule release is impaired in VAMP-7−/− platelets (Figure 2D). This defect in dense granule release could be overcome by exposure of platelets to 5 U/mL of thrombin (supplemental Figure 2A), demonstrating that VAMP-7−/− platelets are not deficient in dense granule cargo. Rather, VAMP-7−/− platelets have a partial defect in agonist-induced dense granule exocytosis that results in a partial aggregation defect.
Dense granule release and platelet aggregation are impaired in VAMP-7−/− platelets. (A) Platelet aggregation (top tracings) and dense granule release (bottom tracings) were monitored in wild-type (black tracings) or VAMP-7−/− platelets (gray tracings) in response to either low concentrations (60 μM) or intermediate concentrations (150 μM) of AYPGKF. (B) Platelet aggregation (top tracings) and dense granule release (bottom tracings) were monitored in wild-type (black tracings) or VAMP-7−/− platelets (gray tracings) in response to either 5 μg/mL or 10 μg/mL collagen. (C) Wild-type (left) and VAMP-7−/− platelets (right) were incubated with a subthreshold concentration of ADP (2 μM) prior to stimulation with AYPGKF (60 μM). Exposure to ADP reverses the aggregation defect in VAMP-7−/− platelets (black tracings). Platelets exposed to ADP alone failed to aggregate (gray tracings). (D) Dense granule release was monitored in wild-type (●) and VAMP-7−/− (○) platelets in response to the indicated concentrations of AYPGKF (**P < .01; n = 3-5). ATP, adenosine triphosphate.
Dense granule release and platelet aggregation are impaired in VAMP-7−/− platelets. (A) Platelet aggregation (top tracings) and dense granule release (bottom tracings) were monitored in wild-type (black tracings) or VAMP-7−/− platelets (gray tracings) in response to either low concentrations (60 μM) or intermediate concentrations (150 μM) of AYPGKF. (B) Platelet aggregation (top tracings) and dense granule release (bottom tracings) were monitored in wild-type (black tracings) or VAMP-7−/− platelets (gray tracings) in response to either 5 μg/mL or 10 μg/mL collagen. (C) Wild-type (left) and VAMP-7−/− platelets (right) were incubated with a subthreshold concentration of ADP (2 μM) prior to stimulation with AYPGKF (60 μM). Exposure to ADP reverses the aggregation defect in VAMP-7−/− platelets (black tracings). Platelets exposed to ADP alone failed to aggregate (gray tracings). (D) Dense granule release was monitored in wild-type (●) and VAMP-7−/− (○) platelets in response to the indicated concentrations of AYPGKF (**P < .01; n = 3-5). ATP, adenosine triphosphate.
VAMP-7 regulates α granule secretion
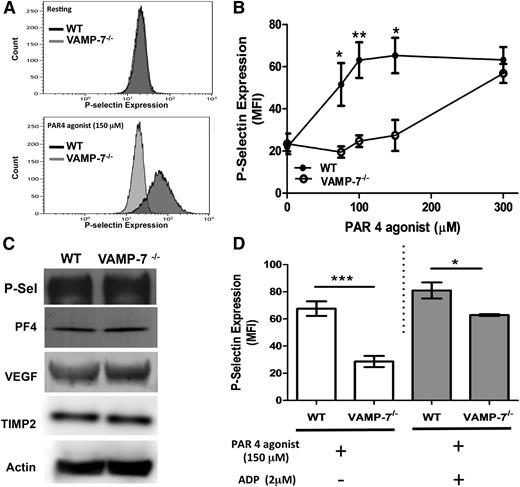
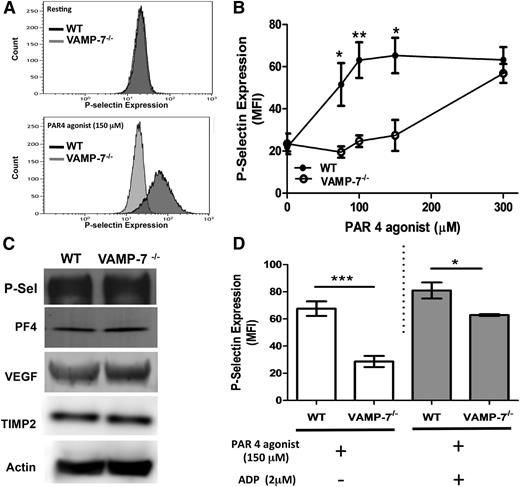
VAMP-7 is present on platelet α granules, and a subpopulation of VAMP-7+ granules move to the platelet periphery during platelet spreading.40 Yet whether or not VAMP-7 functions in α granule exocytosis is not known. To determine whether VAMP-7 participates in α granule exocytosis, we monitored PAR4-mediated P-selectin surface expression in VAMP-7−/− and control platelets. Wild-type platelets showed substantial P-selectin expression upon exposure to 150 μM AYPGKF (Figure 3A). In contrast, little P-selectin expression was observed after exposure of VAMP-7−/− platelets to this concentration of AYPGKF. The defect in α granule release was overcome by a higher concentration of AYPGKF (Figure 3B). Activation-induced surface expression of P-selectin in response to collagen was also impaired in VAMP-7−/− platelets (supplemental Figure 2B). P-selectin content in VAMP-7−/− platelets was similar to that of controls (Figure 3C), indicating that the reduction in activation-induced P-selectin surface expression resulted from impaired exocytosis rather than reduced P-selectin content. Release of the α granule cargo PF4 (also known as CXCL4) was also decreased in VAMP-7−/− platelets stimulated with 60 μM AYPGKF (wild-type, 3.71 ± 0.11 ng of PF4 per 1 × 106 platelets [n = 3]; VAMP-7−/−, 1.55 ± 0.049 ng of PF4 per 1 × 106 platelets [n = 3]; P < .001). Impaired PF4 release did not result from decreased PF4 content because the defect was overcome by stimulation with 5 U/mL of thrombin (wild-type, 4.80 ± 0.11 ng of PF4 per 1 × 106 platelets [n = 3]; VAMP-7−/−, 4.65 ± 0.44 ng of PF4 per 1 × 106 platelets [n = 5]; P = .78), and immunoblot analysis of platelet lysates showed equal amounts of PF4 (Figure 3C). TIMP2 and vascular endothelial growth factor, which colocalize with VAMP-7+ α granules,40 were also equally abundant in wild-type and VAMP-7−/− platelets (Figure 3C). Addition of ADP to VAMP-7−/− platelets partially reversed the defect in AYPGKF-induced P-selectin expression. However, a significant impairment of α granule release was detected even in the presence of ADP (Figure 3D). These results indicate that VAMP-7 deficiency results in a loss of α granule exocytosis that can be overcome by higher concentrations of agonists.
α Granule release is impaired in VAMP-7−/− platelets. (A) P-selectin expression in resting platelets (top) and in response to 150 μM AYPGKF (bottom) was monitored by flow cytometry in wild-type and VAMP-7−/− mice. (B) P-selectin expression in response to the indicated concentrations of AYPGKF was monitored by flow cytometry in wild-type (●) and VAMP-7−/− platelets (○) (*P < .05, **P < .01; n = 3-5). (C) Immunoblot analysis of P-selectin (P-Sel), PF4, vascular endothelial growth factor, TIMP2, and actin (loading control) in wild-type and VAMP-7−/− platelets demonstrates comparable levels of these α granule cargo. (D) Wild-type and VAMP-7−/− platelets were exposed to either vehicle (white bars) or 2 μM ADP (gray bars), subsequently stimulated with 150 μM AYPGKF as indicated, and then evaluated for P-selectin expression by flow cytometry (*P < .05, ***P < .001; n = 3). MFI, median fluorescence intensity.
α Granule release is impaired in VAMP-7−/− platelets. (A) P-selectin expression in resting platelets (top) and in response to 150 μM AYPGKF (bottom) was monitored by flow cytometry in wild-type and VAMP-7−/− mice. (B) P-selectin expression in response to the indicated concentrations of AYPGKF was monitored by flow cytometry in wild-type (●) and VAMP-7−/− platelets (○) (*P < .05, **P < .01; n = 3-5). (C) Immunoblot analysis of P-selectin (P-Sel), PF4, vascular endothelial growth factor, TIMP2, and actin (loading control) in wild-type and VAMP-7−/− platelets demonstrates comparable levels of these α granule cargo. (D) Wild-type and VAMP-7−/− platelets were exposed to either vehicle (white bars) or 2 μM ADP (gray bars), subsequently stimulated with 150 μM AYPGKF as indicated, and then evaluated for P-selectin expression by flow cytometry (*P < .05, ***P < .001; n = 3). MFI, median fluorescence intensity.
Role of VAMP-7 in α granule release in vivo
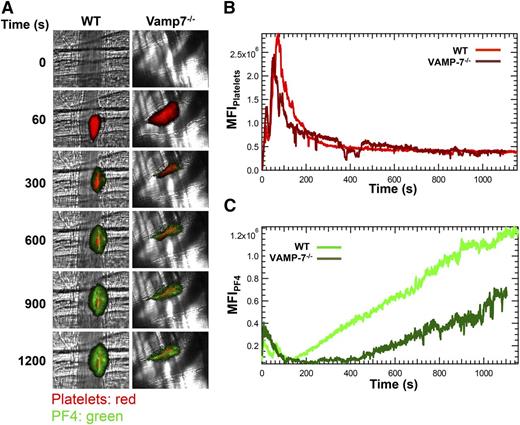
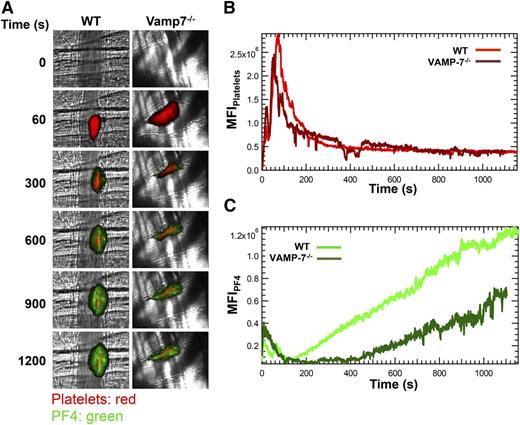
The significant impairment of α granule exocytosis observed in isolated VAMP-7−/− platelets prompted us to assess whether α granule exocytosis is diminished in vivo. Because P-selectin is expressed after activation of either platelets or endothelium, we evaluated the exocytosis of PF4. PF4 is expressed only in platelets and binds back to the thrombus after its release. We monitored both platelet accumulation and PF4 release after laser-induced injury of cremaster arterioles (Figure 4A). Platelet accumulation in VAMP-7−/− mice did not differ significantly from controls (Figure 4B). Consistent with normal platelet accumulation at sites of injury, bleeding times after tail tip amputation were not significantly prolonged in VAMP-7−/− mice (supplemental Figure 3). In contrast, PF4 accumulation at sites of laser injury in VAMP-7−/− mice was decreased to 47% of that of controls (Figure 4C; P ≤ .05) despite the fact that equal number of platelets accumulated at the site of injury (Figure 4B). These results indicate that VAMP-7 deficiency results in impaired α granule secretion in vivo.
α Granule release during thrombus formation in vivo is impaired in VAMP-7−/− mice. (A) Platelet-specific anti-CD42b antibody conjugated to DyLight 649 (0.1 μg/g body weight) and anti-PF4 monoclonal antibody conjugated to Alexa Fluor 488 (0.5 μg/g body weight) were infused into the mice. Representative binarized images of the appearance of fluorescence signals associated with PF4 (green) and platelets (red) after laser-induced vessel wall injury in wild-type and VAMP-7−/− mice are shown. (B) Median integrated platelet fluorescence intensity in wild-type (light red) and VAMP-7−/− mice (dark red). (C) Median integrated PF4 fluorescence intensity at the injury site in wild-type (light green) and VAMP-7−/− mice (dark green) are plotted vs time. Wild-type mice, n = 28; VAMP-7−/− mice, n = 23.
α Granule release during thrombus formation in vivo is impaired in VAMP-7−/− mice. (A) Platelet-specific anti-CD42b antibody conjugated to DyLight 649 (0.1 μg/g body weight) and anti-PF4 monoclonal antibody conjugated to Alexa Fluor 488 (0.5 μg/g body weight) were infused into the mice. Representative binarized images of the appearance of fluorescence signals associated with PF4 (green) and platelets (red) after laser-induced vessel wall injury in wild-type and VAMP-7−/− mice are shown. (B) Median integrated platelet fluorescence intensity in wild-type (light red) and VAMP-7−/− mice (dark red). (C) Median integrated PF4 fluorescence intensity at the injury site in wild-type (light green) and VAMP-7−/− mice (dark green) are plotted vs time. Wild-type mice, n = 28; VAMP-7−/− mice, n = 23.
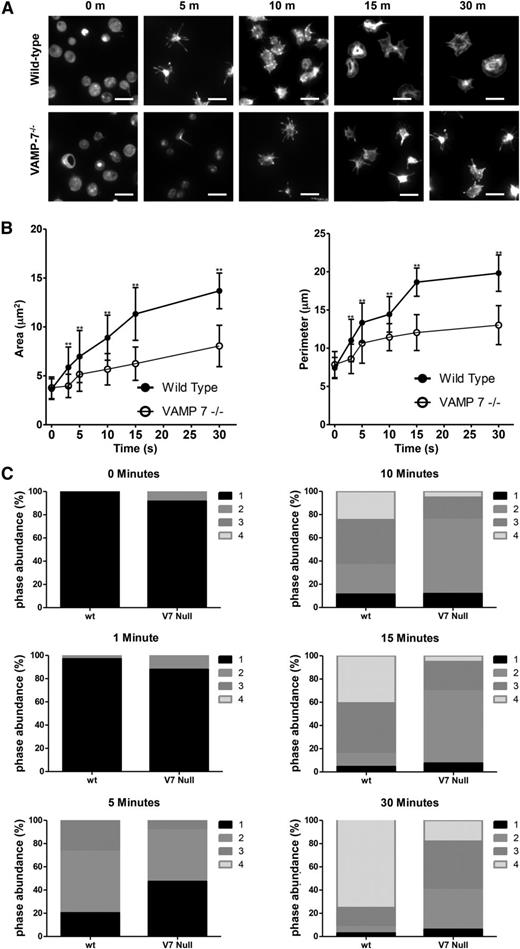
Role of VAMP-7 in platelet spreading
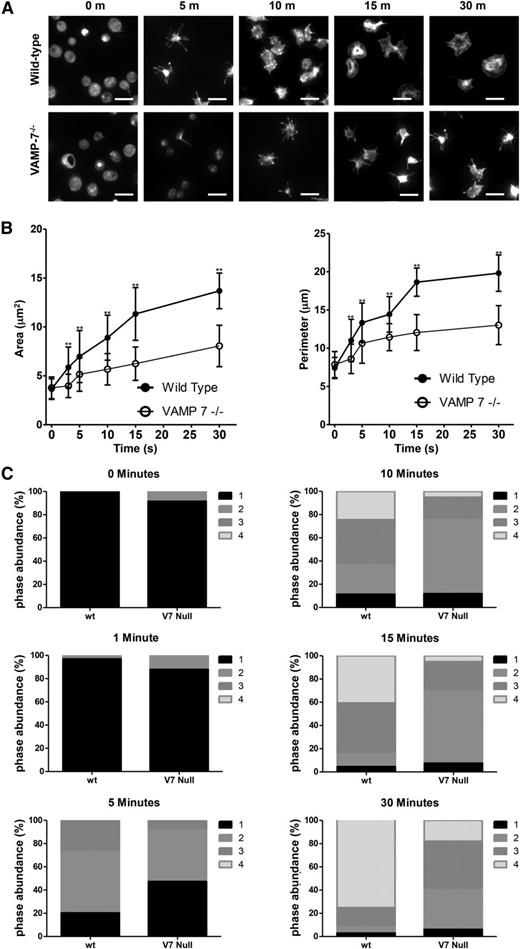
We have previously shown that α granule exocytosis is required for spreading.40 In particular, we used videomicroscopy to identify a subpopulation of α granules that translocate to the periphery of the spread platelet after adhesion. This granule population expressed VAMP-7. In contrast, the majority of platelet granules localized to the granulomere of spread platelets and expressed VAMP-3 and VAMP-8. Based on these observations, we hypothesized that VAMP-7+ α granules contribute to platelet spreading. We studied spreading of VAMP-7−/− platelets to evaluate this hypothesis. VAMP-7−/− and control platelets demonstrate similar areas upon initial contact with collagen; however, control platelets demonstrate a substantial increase in surface area and perimeter as they spread, whereas VAMP-7−/− platelets demonstrate impaired spreading (Figure 5). Specifically, the transition from pseudopodia to lamellipodia is delayed and diminished when analyzed according to the method of Pleines et al (Figure 5C).50 Similarly, spreading of VAMP-7−/− platelets on poly-l-lysine (supplemental Figure 4) or fibrinogen (supplemental Figure 5) was significantly decreased compared to that of control platelets (Figure 5C). Exposure of spreading platelets to ADP did not reverse the defect in platelet spreading observed in VAMP-7−/− platelets (supplemental Figure 6). These results support the supposition that VAMP-7 facilitates platelet spreading.
Spreading on collagen is impaired in VAMP-7−/− platelets. (A) Fluorescence microscopy of wild-type and VAMP-7−/− platelets spread for the indicated amounts of time on collagen and stained with Alexa 546 Phalloidin. Scale bars represent 5 μm. (B) Quantification of surface area (left) and perimeter of spread platelets (right) from wild-type (●) and VAMP-7−/− mice (○) spread on collagen (**P < .01). Error bars represent the standard deviation of measurements from 79 to 384 platelets per time point. (C) Statistical analysis of platelet spreading was performed by quantification of the number of platelets at various stages of spreading as described by Pleines et al.50 According to this method, a value of 1 (black) indicates rounded platelets that lack pseudopodia or lamellipodia; 2 (gray) indicates platelets with pseudopodia only; 3 (dark gray) indicates platelets with pseudopodia and lamellipodia; and 4 (light gray) indicates fully spread platelets with lamellipodia only. This analysis was performed at the indicated time points after seeding on collagen. V7 Null, VAMP-7−/−; wt, wild-type.
Spreading on collagen is impaired in VAMP-7−/− platelets. (A) Fluorescence microscopy of wild-type and VAMP-7−/− platelets spread for the indicated amounts of time on collagen and stained with Alexa 546 Phalloidin. Scale bars represent 5 μm. (B) Quantification of surface area (left) and perimeter of spread platelets (right) from wild-type (●) and VAMP-7−/− mice (○) spread on collagen (**P < .01). Error bars represent the standard deviation of measurements from 79 to 384 platelets per time point. (C) Statistical analysis of platelet spreading was performed by quantification of the number of platelets at various stages of spreading as described by Pleines et al.50 According to this method, a value of 1 (black) indicates rounded platelets that lack pseudopodia or lamellipodia; 2 (gray) indicates platelets with pseudopodia only; 3 (dark gray) indicates platelets with pseudopodia and lamellipodia; and 4 (light gray) indicates fully spread platelets with lamellipodia only. This analysis was performed at the indicated time points after seeding on collagen. V7 Null, VAMP-7−/−; wt, wild-type.
VAMP-7 deficiency does not affect proximal platelet signaling
Loss of VAMP-7 results in defects in multiple platelet functions, including granule secretion, aggregation, and shape change. Our hypothesis is that VAMP-7 acts as a component of the secretory machinery to mediate a terminal event in membrane fusion and granule release. Alternatively, VAMP-7 could participate in upstream signaling events that are required for these multiple signaling pathways. To assess this possibility, we evaluated several proximal signaling events in VAMP-7−/− platelets. To investigate whether defective release of calcium from internal stores in VAMP-7−/− platelets accounted for their impaired function, we evaluated PAR4-mediated [Ca2+]i flux. PAR4-mediated [Ca2+]i flux in VAMP-7−/− platelets was not statistically different from that in wild-type controls at any of the concentrations tested, indicating normal proximal signaling in the VAMP-7−/− platelets (Figure 6A). PAR4-dependent activation of serine/threonine and tyrosine phosphorylation was also similar in VAMP-7−/− and control platelets (Figure 6B). In wild-type and VAMP-7−/− platelets, αIIbβ3 was expressed at similar levels (Figure 6C; supplemental Figure 7A; P = .97). Activation of αIIbβ3, as detected by the Jon/A antibody after stimulation with 150 μM SFLLRN, was also similar in wild-type and VAMP-7−/− platelets (Figure 6D; supplemental Figure 7B; P = .3). These observations suggest that loss of VAMP-7 does not result in an upstream signaling defect.
Proximal signaling mechanisms are not impaired in VAMP-7−/− platelets. (A) [Ca2+]i flux in wild-type and VAMP-7−/− mice incubated with fura-2 was monitored after incubation with the indicated concentrations of AYPGKF. (B) Immunoblot analysis using an anti-phosphoserine/phosphothreonine antibody (PS/PT; left) or an anti-phosphotyrosine antibody (PY) of lysates (right) from wild-type and VAMP-7−/− (V7−/−) platelets before (Resting) and after (Activated) stimulation with 150 μM AYPGKF. (C) Wild-type (top) and VAMP-7−/− platelets (bottom) were stained with anti-CD41 antibody and evaluated by flow cytometry. (D) Wild-type (top) and VAMP-7−/− platelets (bottom) were incubated in the presence of vehicle (Resting; left) or 150 μM AYPGKF (Activated; right), stained with Jon/A antibody, and evaluated by flow cytometry.
Proximal signaling mechanisms are not impaired in VAMP-7−/− platelets. (A) [Ca2+]i flux in wild-type and VAMP-7−/− mice incubated with fura-2 was monitored after incubation with the indicated concentrations of AYPGKF. (B) Immunoblot analysis using an anti-phosphoserine/phosphothreonine antibody (PS/PT; left) or an anti-phosphotyrosine antibody (PY) of lysates (right) from wild-type and VAMP-7−/− (V7−/−) platelets before (Resting) and after (Activated) stimulation with 150 μM AYPGKF. (C) Wild-type (top) and VAMP-7−/− platelets (bottom) were stained with anti-CD41 antibody and evaluated by flow cytometry. (D) Wild-type (top) and VAMP-7−/− platelets (bottom) were incubated in the presence of vehicle (Resting; left) or 150 μM AYPGKF (Activated; right), stained with Jon/A antibody, and evaluated by flow cytometry.
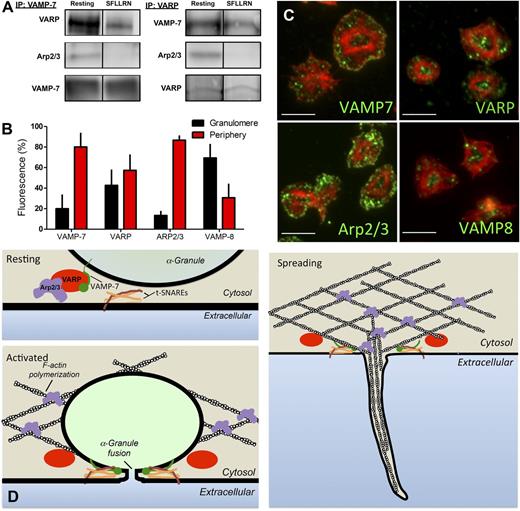
Association of VAMP-7 with platelet VARP and Arp2/3
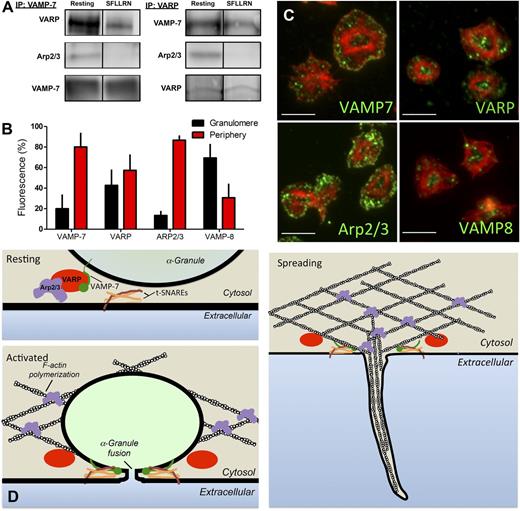
The observation that spreading is defective in VAMP-7−/− platelets (Figure 5) suggests a role for VAMP-7 in cytoskeletal dynamics as well as in membrane fusion. VAMP-7 is unique among VAMP isoforms in that it contains an N-terminal longin domain capable of interacting with adaptor proteins and cytoskeletal components. To identify VAMP-7 binding partners in platelets, immunoprecipitation of VAMP-7 was performed, and the immunoprecipitates were evaluated by mass spectroscopy. Evaluation of VAMP-7 binding proteins demonstrated an interaction with VARP (an adaptor protein that has not previously been described in platelets) and with Arp2/3 (an actin-binding protein previously shown to function in platelet actin reorganization).51,52 Immunoprecipitation of VAMP-7 followed by immunoblot analysis using anti-VARP and anti-Arp2/3 antibodies confirmed an association of VARP and Arp2/3 with VAMP-7 (Figure 7A). The association of VARP and Arp2/3 with VAMP-7 decreased after stimulation of platelets by SFLLRN. Immunoprecipitation of VARP followed by mass spectroscopy indicated an association of VARP with VAMP-7 and Arp2/3. Immunoprecipitation of VARP followed by immunoblot analysis confirmed this association and demonstrated decreased association of VAMP-7 and Arp2/3 with VARP after platelet activation, consistent with results obtained with immunoprecipitation of VAMP-7 (Figure 7A). These studies suggest an activation-sensitive association of VAMP-7, VARP, and Arp2/3.
VARP associates with VAMP-7 and Arp2/3 in an activation-dependent manner. (A) VAMP-7 and VARP were immunoprecipitated (IP) from human platelets before or after exposure to 5 μM SFLLRN. Immunoprecipitated proteins were separated by sodium dodecyl sulfate−polyacrylamide gel electrophoresis and evaluated for VARP, Arp2/3, and VAMP-7 by immunoblot analysis. (B) Double immunofluorescence microscopy of actin and either VAMP-7, VARP, Arp2/3, or VAMP-8 was performed, and images were analyzed as previously described40 to demarcate the granulomere and periphery of spread platelets. The percentage of VAMP-7, VARP, Arp2/3, and VAMP-8 fluorescence in the granulomere and periphery was quantified. Measurements represent the standard deviation of 25 individual platelet measurements per condition. (C) Representative images of double immunofluorescence microscopy of actin and VAMP-7, VARP, Arp2/3, or VAMP-8. Scale bars represent 5 microns. (D) Model of putative role for VARP in linking platelet granule exocytosis and actin polymerization. In the resting state (top left), VARP binds VAMP-7 and Arp2/3, localizing the granule exocytosis machinery and the actin polymerization machinery to the same location and maintaining them in an inactive state. Following platelet activation (bottom left), VAMP-7 and Arp2/3 are released from VARP. VAMP-7 interacts with target (t)-SNAREs on the plasma membrane and Arp2/3 functions in actin reorganization. Fusion of granules with the plasma membrane provides extra membrane to cover growing actin structures during spreading (right).
VARP associates with VAMP-7 and Arp2/3 in an activation-dependent manner. (A) VAMP-7 and VARP were immunoprecipitated (IP) from human platelets before or after exposure to 5 μM SFLLRN. Immunoprecipitated proteins were separated by sodium dodecyl sulfate−polyacrylamide gel electrophoresis and evaluated for VARP, Arp2/3, and VAMP-7 by immunoblot analysis. (B) Double immunofluorescence microscopy of actin and either VAMP-7, VARP, Arp2/3, or VAMP-8 was performed, and images were analyzed as previously described40 to demarcate the granulomere and periphery of spread platelets. The percentage of VAMP-7, VARP, Arp2/3, and VAMP-8 fluorescence in the granulomere and periphery was quantified. Measurements represent the standard deviation of 25 individual platelet measurements per condition. (C) Representative images of double immunofluorescence microscopy of actin and VAMP-7, VARP, Arp2/3, or VAMP-8. Scale bars represent 5 microns. (D) Model of putative role for VARP in linking platelet granule exocytosis and actin polymerization. In the resting state (top left), VARP binds VAMP-7 and Arp2/3, localizing the granule exocytosis machinery and the actin polymerization machinery to the same location and maintaining them in an inactive state. Following platelet activation (bottom left), VAMP-7 and Arp2/3 are released from VARP. VAMP-7 interacts with target (t)-SNAREs on the plasma membrane and Arp2/3 functions in actin reorganization. Fusion of granules with the plasma membrane provides extra membrane to cover growing actin structures during spreading (right).
The association of VAMP-7 with VARP and Arp2/3 could provide a mechanism whereby membrane fusion is coupled to actin reorganization to provide auxiliary membrane to cover growing actin structures. The coupling of membrane fusion to actin reorganization would be expected to occur in the periphery of the spreading platelet, where pseudopodia and lamellipodia formation occurs. To assess the localization of VAMP-7, VARP, and Arp2/3 in adherent platelets, platelets were spread on poly-l-lysine and subsequently stained with phalloidin to facilitate the distinction between the central granulomere and the platelet periphery, as previously described.40 VAMP-7 and Arp2/3 demonstrated localization primarily to the platelet periphery (Figure 7B-C). The localization of VARP was divided approximately evenly between granulomere and periphery. In contrast, VAMP-8 localized primarily to the platelet granulomere (Figure 7B-C), as has been demonstrated previously for the majority of granule proteins.40 Immunofluorescence staining of spread platelets demonstrated that VAMP-7 and VARP colocalized in spread platelets (supplemental Figure 8). Similarly, VAMP-7 partially colocalized with Arp2/3 in the spread platelet, as did VARP and Arp2/3 (supplemental Figure 8). The activation-sensitive association of VAMP-7, VARP, and Arp2/3 in the periphery of the spreading platelet could provide a mechanism that links membrane fusion to actin polymerization (Figure 7D).
Discussion
These studies demonstrate a role for VAMP-7 in platelet granule exocytosis and spreading. VAMP-7 shares significant amino acid sequence similarity to VAMP-8 in the SNARE domain and is more closely related to VAMP-8 than to VAMP-2 or VAMP-3. Both VAMP-7 and VAMP-8 function in late membrane fusion events, including the trafficking of granules from endosomes to the cell surface.53 VAMP-7 and VAMP-8, but not VAMP-2 or VAMP-3, function in the exocytosis of mast cell granules, which (like platelet granules) are released by compound exocytosis.54 Unlike VAMP-8, however, VAMP-7 contains an N-terminal extension that enables VAMP-7 to associate with several binding partners with which VAMP-8 does not interact.28,29 This longin domain influences both the regulation and function of VAMP-7,55 allowing it to act at the nexus of cytoskeletal remodeling and membrane fusion.
Loss of VAMP-7 results in only a partial defect in granule release, consistent with previous observations that VAMP-8 serves a major role in platelet granule exocytosis.24 Defects in exocytosis are not secondary to impaired granule formation, as indicated by normal platelet morphology in VAMP-7 nulls. Normal cargo content is indicated by the observation that full secretion is observed in response to high agonist concentrations (Figures 2 and 3) and with immunoblot analysis of α granule contents (Figure 3). The fact that agonist-induced [Ca2+]i flux and serine/threonine and tyrosine protein phosphorylation are similar in VAMP-7−/− and control platelets suggests that proximal signaling events are not affected by the loss of VAMP-7 (Figure 6). The phenotype of impaired granule release and aggregation in the absence of defects in proximal signaling events has also been observed in VAMP-8−/− and Munc13-4−/− platelets.12,24 Impairment of PF4 release after arteriolar injury in VAMP-7−/− mice despite that fact that platelet accumulation is normal provides further evidence that VAMP-7 is required for normal α granule exocytosis and shows that its loss results in a partial defect in granule release in vivo. The fact that platelet accumulation is not impaired during thrombus formation distinguishes the phenotype in VAMP-7−/− mice from that of VAMP-8−/− mice, as well as from Nbeal2−/− mice, which are deficient in α granules and demonstrate a defect in thrombus formation.5,8 In addition, VAMP-7−/− mice do not demonstrate prolonged bleeding with tail clip (supplemental Figure 3). The observation that aggregation is not impaired in response to 150 μM PAR4 agonist (Figure 2), whereas α granule release is nearly absent under these conditions (Figure 3), is consistent with the in vivo observation that PF4 release is impaired despite normal platelet accumulation (Figure 4). Recent studies using Nbeal2-null mice indicate that α granules serve functions beyond hemostasis and thrombosis, such as contributing to tumor metastases.9 Whether interference with VAMP-7 could interfere with such activities without affecting hemostasis remains to be determined.
With its N-terminal longin domain, VAMP-7 is a candidate v-SNARE for linking cytoskeletal elements to the SNARE machinery and thus orchestrating membrane fusion events required for platelet spreading. We have previously shown that the subpopulation of α granules that translocates to the platelet periphery during platelet spreading expresses VAMP-7.40 We now demonstrate that spreading is decreased in VAMP-7−/− platelets. Our hypothesis is that VAMP-7+ granules translocate to the periphery of the spreading platelet and fuse with the plasma membrane to provide membrane for directed exocytosis during spreading. VAMP-7 functions in directed granule exocytosis in several cell types that use granules as a source of auxiliary membrane. In macrophages, VAMP-7 functions in delivering granules to the phagocytotic cup.33,34 In neurons, VAMP-7 mediates membrane fusion required for tubulovesicular structures at the leading edge of elongating dendrites and axons that support neurite outgrowth.30-32 We now demonstrate a role for VAMP-7 in delivering membrane to the platelet periphery during spreading.
Although platelet granule exocytosis has largely been studied in suspension platelets, physiologic granule release during thrombosis and inflammation occurs in adherent platelets, where cytoskeletal remodeling occurs concurrently with exocytosis. Real-time imaging of platelets adhering to matrices under flow conditions demonstrates that individual platelets form elongated membrane tethers that can extend up to 250 μm.56-58 Platelet membrane projections have also been identified in vivo during thrombus formation.56 Although the open canalicular system undoubtedly provides some of the reserve membrane required for the formation of these tethers, directed granule exocytosis of α granules, particularly of VAMP-7+ granules, could provide a rapid means of directed membrane delivery to a growing tether.
The molecular mechanisms that enable dynamic coordination of cytoskeletal and membrane remodeling in platelets are poorly understood. Our studies demonstrate that VAMP-7 serves an important link between exocytosis and actin reorganization. We now show that VAMP-7 associates with VARP and Arp2/3 in platelets. VARP is a multidomain adaptor protein previously shown to bind VAMP-7 and maintain it in an inactive conformation.59 VARP is required for stimulus-induced dendrite formation in melanocytes60 and neurite outgrowth in neurons, where it recruits the molecular motor Kif5, tethering factor GolginA4, and plakin MACF-1.61 Arp2/3 is required for actin polymerization during platelet spreading and promotes orthogonal branching of actin filaments.52 A pathway involving Rac1-dependent signaling through Arp2/3 and coordinating the activity of VAMP-7 has been described for neurite outgrowth.62 Evaluation of neurite outgrowth has also demonstrated that under different conditions, Cdc42 can control exocytosis of VAMP-7–containing vesicles to cover growing actin structures.32 The role of the VAMP-7:VARP:Arp2/3 complex in linking exocytosis with actin reorganization in platelets and its control by upstream signaling pathways require additional characterization. VARP could localize this F-actin–generating machinery with granule exocytosis through VAMP-7 (Figure 7D). Further studies will be required to assess this hypothesis and detail the role of Arp2/3 and VARP in linking platelet cytoskeletal remodeling to granule exocytosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (HL112809) and the American Heart Association (0840043N), and by the Foundation for Women’s Wellness.
Authorship
Contribution: S.K. designed and performed the exocytosis and spreading assays, performed and analyzed the mass spectroscopy and immunoprecipitation studies, designed the figures, and assisted with writing and editing the manuscript; C.G.P. performed the spreading assays, performed and supervised the microscopy, and edited the manuscript; J.L.F.-T. performed the in vivo studies; O.A. performed the [Ca2+]i flux studies; L.D. and T.G. provided VAMP-7−/− mice and anti-VAMP-7−/− antibodies and edited the manuscript; and R.F. initiated the project, led the project team, designed the experiments, analyzed the results, designed the figures, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Flaumenhaft, Division of Hemostasis and Thrombosis, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; e-mail: rflaumen@bidmc.harvard.edu.

![Figure 6. Proximal signaling mechanisms are not impaired in VAMP-7−/− platelets. (A) [Ca2+]i flux in wild-type and VAMP-7−/− mice incubated with fura-2 was monitored after incubation with the indicated concentrations of AYPGKF. (B) Immunoblot analysis using an anti-phosphoserine/phosphothreonine antibody (PS/PT; left) or an anti-phosphotyrosine antibody (PY) of lysates (right) from wild-type and VAMP-7−/− (V7−/−) platelets before (Resting) and after (Activated) stimulation with 150 μM AYPGKF. (C) Wild-type (top) and VAMP-7−/− platelets (bottom) were stained with anti-CD41 antibody and evaluated by flow cytometry. (D) Wild-type (top) and VAMP-7−/− platelets (bottom) were incubated in the presence of vehicle (Resting; left) or 150 μM AYPGKF (Activated; right), stained with Jon/A antibody, and evaluated by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/5/10.1182_blood-2014-12-618744/4/m_651f6.jpeg?Expires=1767726713&Signature=JnNFkdpKhl-5Fv8FLz4mgx1jr~RkoVunFCL0x1kPvGpOWO-0-HnJjvIhWWOzJuR3yG6~tM048WFC6kWZuEvLkkmj12ErCfsLuXI0vVclghgn0HBrginAsnl8wdhQ6MM-NTk22wWCFWE5uvKBBerJFI5Ve6T9FXPV~Qg7ffGE-QNL1x8wRIrT4cpAS-9COIJWl2wOksw~tCoRFusEMEtZvq3mGnO~KAJR4HbrCrM-GcmCVAk-vJlx5qbqoK7lZfkT2jCzzf-QZvjEZCE-jaAhsjGAM7eBlzeK-wN3EaHTaL9tyNVkUCfQNOaCN96fArg6LdsiaYm6EjWDcWdPaQ2L4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Proximal signaling mechanisms are not impaired in VAMP-7−/− platelets. (A) [Ca2+]i flux in wild-type and VAMP-7−/− mice incubated with fura-2 was monitored after incubation with the indicated concentrations of AYPGKF. (B) Immunoblot analysis using an anti-phosphoserine/phosphothreonine antibody (PS/PT; left) or an anti-phosphotyrosine antibody (PY) of lysates (right) from wild-type and VAMP-7−/− (V7−/−) platelets before (Resting) and after (Activated) stimulation with 150 μM AYPGKF. (C) Wild-type (top) and VAMP-7−/− platelets (bottom) were stained with anti-CD41 antibody and evaluated by flow cytometry. (D) Wild-type (top) and VAMP-7−/− platelets (bottom) were incubated in the presence of vehicle (Resting; left) or 150 μM AYPGKF (Activated; right), stained with Jon/A antibody, and evaluated by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/5/10.1182_blood-2014-12-618744/4/m_651f6.jpeg?Expires=1767874325&Signature=FgqbsrZwGAKP2ufFR4icuRXhkD~vQhhEiCAxfRe~bimorqms3RFzFNSfkIq4wZHKHvNB5PpO9cqgyO42-pQyXg7S2ZBSaR16rK~WowvX71HogKwHe~0AmE3c75I41tpBQQAaJ6mLCMJ-a0S1kIpOs4Hid21EZgQla~jC4xSln4nOszez0RmZDHs5GFuvNJG2nMRGfyje~lYv5G35AD0bDnoOkA2qSqxMr3-25nA0X1SbW5HK6k6ZY5oVDxm1RN6tgL7-RCKKR5hUR1d2jYERbfFN~CGWx~1PiY43P7l-3L3Qa9xw4pDCrQpNjzYnp536fB-hC2CLkwa7HeFsjTiHDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)