In this issue of Blood, 2 articles test fundamental hypotheses relating intravascular hemolysis to sickle cell disease (SCD) pathogenesis. Detterich et al confirm a role for hemolysis and cell-free plasma hemoglobin (Hb) in pulmonary and systemic endothelial dysfunction in humans.1 Almeida et al show in mice that hemolysis induces inflammation that is caused by nitric oxide (NO) scavenging and ameliorated by NO donors and the NO-donor properties of hydroxyurea (HU).2
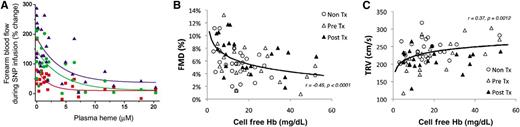
Relationship between cell-free plasma Hb levels (levels reported in terms of heme) and (A) blood flow responses to intra-arterial infusions of sodium nitroprusside, showing that patients with higher plasma cell-free Hb have blunted vasodilation responses to NO donors (reproduced with permission from Reiter et al4 ), (B) flow-mediated vasodilation, a measure of endothelial NO production from NO synthase during shear-stress, which is blunted in patients with higher plasma cell-free Hb levels (see Figure 4 in the article by Detterich et al that begins on page 703), and (C) the TR jet velocity, a noninvasive estimate of pulmonary artery systolic pressure that is higher in patients with higher levels of plasma cell-free Hb (see Figure 3 in the article by Detterich et al that begins on page 703).
Relationship between cell-free plasma Hb levels (levels reported in terms of heme) and (A) blood flow responses to intra-arterial infusions of sodium nitroprusside, showing that patients with higher plasma cell-free Hb have blunted vasodilation responses to NO donors (reproduced with permission from Reiter et al4 ), (B) flow-mediated vasodilation, a measure of endothelial NO production from NO synthase during shear-stress, which is blunted in patients with higher plasma cell-free Hb levels (see Figure 4 in the article by Detterich et al that begins on page 703), and (C) the TR jet velocity, a noninvasive estimate of pulmonary artery systolic pressure that is higher in patients with higher levels of plasma cell-free Hb (see Figure 3 in the article by Detterich et al that begins on page 703).
Between 2002 and 2005, we published a series of studies characterizing the role of hemolysis in sickle cell vasculopathy, suggesting that the release of red cell contents into plasma inhibits NO signaling and impairs endothelial function.3-7 Specifically, cell-free plasma Hb reacts with and scavenges NO via the fast and irreversible dioxygenation reaction to form nitrate. Hemolysis also releases arginase-1, activates vascular oxidases, and increases reactive oxygen species (ROS) formation.5,7 Recent studies have also identified a critical role for downstream Hb oxidation and release of free heme, which activates toll-like receptor 4 and inflammasome pathways.8 Physiological and epidemiological studies demonstrated that cell-free plasma Hb and clinical markers of hemolysis were associated with measures of endothelial dysfunction and noninvasive and invasive measures of pulmonary hypertension.9,10 Interestingly, these early studies also suggested gender divergence, with male sickle cell patients suffering higher rates of hemolysis and more impaired endothelial function.3
This model of disease mechanism has been referred to as the “hyperhemolysis paradigm” and proposed to constitute a mechanism for human disease, relevant to SCD, malaria, and iatrogenic hemolytic processes like the transfusion of aged stored blood.5 However, this has remained controversial with editorial commentary challenging causality, interpretation of biomarkers of hemolysis, and the prevalence and importance of pulmonary hypertension.11 Of relevance to this controversy, 2 new studies now evaluate these hypotheses in humans and in mouse models of acute hemolysis and SCD.1,2
Detterich et al explored the relationship between noninvasive measures of endothelial function and NO production from the endothelium, using flow-mediated dilation (FMD).1 This physiological response to brief forearm ischemia followed by metabolic vasodilation and flow-mediated conduit artery dilation is considered a specific test of endothelial NO synthase activity. The authors report that a noninvasive measure of pulmonary artery systolic pressure, the tricuspid regurgitant (TR) jet velocity, correlates with impaired FMD (P < .0001), and both correlate significantly with the levels of cell-free plasma Hb. Interestingly, most SCD patients with a TR jet velocity >2.7 m/s had endothelial dysfunction, defined by a measured FMD <6%. Patients with elevated levels of cell-free plasma Hb >2 standard deviations above the mean for controls had elevated TR jet velocity (relative risk [RR], 3.47; 95% confidence interval [CI], 0.88-13.65; P = .03) and decreased FMD (RR 2.51; 95% CI, 1.15-5.47; P = .003). On multivariate statistical analysis, both TR jet velocity and FMD were associated with traditional biomarkers of hemolysis and high cell-free plasma Hb levels. Interestingly, higher FMD also had a positive association with gender (female), as previously described.3
The investigators evaluated the effects of acute and chronic transfusion on these end points. Acute transfusion had minimal effects on TR jet velocity and cell-free plasma Hb, but did acutely improve FMD. Chronically transfused adult patients had lower TR jet velocity and higher FMD, suggesting improved vascular function over time. The finding that TR jet velocity is improved in the group with chronic transfusion is of significant interest as TR jet velocity is a biomarker of disease severity, associated with an increased risk of having pulmonary hypertension defined by right heart catheterization, and an independent risk factor for early death.9,10,12
In the second study, Almeida et al report that acute intravascular hemolysis induced by hypotonic water infusion in mice produces acute NO metabolism to nitrate and acute inflammation, as measured by decreased leukocyte rolling, and increased adhesion and extravasation. Interestingly, this effect is phenocopied by acute NO inhibition using 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) and blocked by an NO donor. It is now appreciated that heme can activate inflammation, so they tested the contributions of Hb and heme by treating mice with heme, haptoglobin, and hemopexin. Heme also induced inflammation but this was delayed in this model. Furthermore, haptoglobin completely inhibited the inflammatory response, whereas hemopexin only partially blocked inflammation. Finally, the investigators tested acute HU dosing. Interestingly, HU acutely blocked the inflammatory response to hemolysis; this effect was abolished by the NO scavenger PTIO and by the soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), consistent with reported NO donor properties of HU.6
Both of these studies raise provocative questions about new therapeutic approaches for SCD patients utilizing our older therapies, transfusion, and HU. Considering the robust effects of acute transfusion on improving FMD, should this be considered for acute vaso-occlusive crisis? Should we explore chronic transfusion therapy for our patients at highest risk of developing pulmonary hypertension and death? There are a number of biomarkers that identify adult SCD patients at the highest risk of death, including TR jet velocity, plasma levels of N-terminal pro-brain natriuretic peptide, and pulmonary hypertension defined by right heart catheterization.13 The current findings support the prioritization of clinical trials of chronic transfusion targeting these at-risk groups. Finally, HU therapy is often held during hospitalization for vaso-occlusive pain crisis and acute chest syndrome because patients are not able to tolerate oral drug dosing or because of perceived lack of efficacy in this setting. Should we consider trials of IV HU therapy or more aggressive oral therapy during routine hospitalization?
These studies in aggregate support a model for SCD pathogenesis initiated by sickle Hb polymerization, which alters red cell rheological properties and leads to hemolysis. Downstream organ injury is caused by microvascular vaso-occlusion, characterized by red cell and leukocyte adhesion, inflammation, and ischemia-reperfusion pathophysiology. Acute and chronic hemolysis dysregulates the endothelial redox balance (decrease in NO/ROS) and impairs endothelial function, causing progressive systemic and pulmonary vasculopathy. Failed clinical therapies that modulate the hemolytic spectrum of disease by amplifying the NO signaling axis using inhaled NO and phosphodiesterase type 5-inhibitors remain a challenge for the field, perhaps to be overcome with old therapies, like transfusion and HU, or with new generation therapies targeting the NO-cyclic guanosine monophosphate axis, like the direct soluble guanylate cyclase stimulators and activators.
Conflict-of-interest disclosure: The author declares no competing financial interests.