Key Points
Patients with relapsed or refractory transformed indolent lymphoma and DLBCL have similar outcomes with salvage therapy and ASCT.
This therapy should be considered the standard of care for previously treated transformed indolent lymphoma.
Abstract
The treatment of transformed indolent lymphoma (TRIL) often includes salvage chemotherapy (SC) and autologous stem cell transplant (ASCT). NCIC CTG LY12 is a randomized phase 3 trial comparing gemcitabine, dexamethasone, and cisplatin (GDP) with dexamethasone, cytarabine, and cisplatin (DHAP) before ASCT. This analysis compares the results of SC and ASCT for TRIL with de novo diffuse large B-cell lymphoma (DLBCL). Six-hundred nineteen patients with relapsed/refractory aggressive non-Hodgkin lymphoma were randomized to GDP or DHAP; 87 patients (14%) had TRIL and 429 (69%) had DLBCL. The response rate to SC was 47% in TRIL and 45% in DL (P = .81). Transplantation rates were similar: TRIL 53% and DL 52% (P = 1.0). With a median follow-up of 53 months, 4 year overall survival was 39% for TRIL and 41% for DL (P = .78); 4 year event-free survival (EFS) was 27% for TRIL and 27% for DL (P = .83). Post-ASCT, 4-year EFS was 45% for TRIL and 46% for DL. Histology (TRIL or DL) was not a predictor of any outcome in multivariate models. Patients with relapsed or refractory TRIL and DLBCL have similar outcomes with SC and ASCT; this therapy should be considered the standard of care for patients with TRIL who have received prior systemic chemotherapy. NCIC CTG LY12 is registered at ClinicalTrials.gov as #NCT00078949.
Introduction
Histologic transformation in patients with indolent non-Hodgkin lymphoma (iNHL) involves the development of an aggressive histology lymphoma after a prior diagnosis of iNHL. In follicular lymphoma, the risk of transformation has been estimated to be ∼2% to 3% per year from the initial diagnosis, leading to a cumulative risk of 25% to 30% at 10 years.1-3 Historical cohort data in patients with transformed indolent lymphoma (TRIL) have typically reported poor overall survival (OS) despite combination chemotherapy, with most patients dying from progressive disease.1-5
Treatment of transformed indolent lymphoma has been variable and generally described in retrospective patient cohorts. Given the perception of poor outcome with standard-dose chemotherapy, selected patients (typically younger and without prohibitive comorbidities) are treated with aggressive strategies involving autologous stem cell transplantation (ASCT) or allogeneic stem cell transplantation.6-16 A retrospective case-control comparison of outcomes of ASCT in patients with TRIL or with de novo intermediate or high-grade lymphoma published more than 10 years ago by the European Society for Blood and Marrow Transplantation (EBMT) suggested similar outcomes in these groups.14 With ASCT defined as a standard therapy for relapsed diffuse large B-cell lymphoma (DLBCL) and other aggressive histology NHL based on the randomized Parma trial,17 the EBMT review concluded that ASCT should be considered for TRIL.
Reports of transplant outcomes for TRIL are limited by the retrospective nature of the majority of these reports and the inherent selection bias of solely reporting transplanted patients. The proportion of patients able to respond to aggressive salvage chemotherapy, mobilize peripheral blood stem cells (PBSCs), and undergo subsequent transplantation has not been well defined or reported in prospective studies. This analysis was designed to help address the lack of prospective data in this area.
The NCIC Clinical Trials Group (NCIC CTG) LY.12 study was a randomized controlled trial designed to compare gemcitabine, dexamethasone, and cisplatin (GDP) with dexamethasone, cytarabine, and cisplatin (DHAP) as salvage chemotherapy before autologous transplantation (first randomization), and to evaluate the efficacy of posttransplantation treatment with the anti-CD20 antibody rituximab (second randomization). The results of the first randomization demonstrated that GDP was noninferior to DHAP in terms of response rate and resulted in a similar percentage of patients proceeding to ASCT, with significantly less toxicity and need for hospitalization.18 Here, we present the outcome of a planned subset analysis of patients with TRIL enrolled onto this study compared with patients with de novo DLBCL.
Methods
The NCIC Clinical Trials Group LY.12 study was an international randomized controlled trial conducted in 26 Canadian and 10 American centers, and 1 Australian center, and, in collaboration with the Gruppo Italiano Studio Linfomi (GISL), 18 centers in Italy. Details of the trial design, conduct, and financial support are reported elsewhere.18 The trial was approved by the research ethics boards of all participating centers, and written informed consent was provided by all participants.
Patient selection for this subset analysis
From the entire LY12 cohort, we identified patients with biopsy-proven indolent B-cell NHL and simultaneous or subsequent histologic evidence of transformation to aggressive histology B-cell lymphoma for inclusion in this preplanned subset analysis. The following preceding indolent lymphomas were included: follicular lymphoma grade 1-3A, marginal-zone lymphoma, lymphoplasmacytic lymphoma, and small lymphocytic lymphoma/chronic lymphocytic leukemia. The following aggressive transformation histologies were included: DLBCL, Burkitt lymphoma, B-cell NHL with features intermediate between DLBCL and Burkitt lymphoma, and lymphoblastic lymphoma.19 Pathology was reviewed at each center by a local reference pathologist at the time of TRIL diagnosis or treatment and pathology reports both at the time of initial diagnosis and subsequent transformation were reviewed by a physician at the operations office of NCIC CTG.
Patients with TRIL were eligible for randomization if they had received no more than three prior systemic treatments (before an amendment early in the trial, there was no limit to prior systemic therapy). Baseline assessments included physical examination; standard laboratory testing; computed tomography (CT) scanning of the chest, abdomen, and pelvis; bone marrow biopsy; and, if indicated, cerebrospinal fluid analysis. Eligible patients were required to have measurable disease by CT scan or physical examination, an Eastern Cooperative Oncology Group Performance Status of 0-3,20 and acceptable hematologic and biochemical parameters. Patients were excluded if they previously received treatment with cisplatin, cytarabine, or gemcitabine, had central nervous system involvement with lymphoma, a history of HIV infection, or a medical condition that would interfere with safe delivery of protocol chemotherapy.
At the time of randomization, eligible patients were stratified by International Prognostic Index at trial entry,21 response to primary therapy, immunophenotype (B vs T cell), study center, and prior rituximab exposure. Centers were permitted to use local practices and policies for stem-cell mobilization and transplantation and supportive care.
Response assessment
The primary outcome for the first randomization of LY12 was response (complete, unconfirmed complete, or partial response) after 2 cycles of therapy, according to International Workshop Criteria22 ; fluorodeoxyglucose positron emission tomography was not used to assess response. OS was calculated from the date of randomization to the date of death from any cause; event-free survival (EFS) was calculated from the date of randomization to the date of disease progression, relapse after objective response, initiation of new lymphoma therapy, or death from any cause. Response rate, EFS, and OS were evaluated using data from an intention-to-treat population. Adverse events were graded according to National Cancer Institute Common Toxicity Criteria Version 2.0.22
Statistical analysis
The primary analysis of LY12 met its noninferiority end point; there was no significant association between study arm (GDP vs DHAP) and the primary end point of response, or the secondary end points of EFS or OS. Patients with TRIL or DLBCL receiving either GDP or DHAP were combined for this secondary analysis. The χ2 test23 was used to compare the rates of response, transplantation, adverse events, and successful stem cell mobilization between patients with TRIL and de novo DLBCL. The life-table method of Kaplan and Meier24 was used to calculate EFS and OS, and groups were compared using the log-rank test.25 All analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
During the period from August 2003 to November 2011, 619 patients were randomized in LY.12. Eighty-seven patients had TRIL at the time of study enrollment (43 patients randomized to GDP and 44 to DHAP) and 428 patients were enrolled with DLBCL (220 randomized to GDP and 209 to DHAP; Table 1). The remainder had lymphomas of T or natural killer cell origin. The indolent histology in the TRIL group was follicular lymphoma in 78 patients (90%) and marginal zone lymphoma in 4 (5%), with the other cases including unclassified and lymphoplasmacytic lymphoma (n = 5). In patients with an indolent lymphoma, the time to diagnosis of DLBCL ranged from 0 to 205 months, with a median of 29 months. The median age was 56 in both the TRIL and DLBCL groups, with 30% of patients in both groups over the age of 60 years. Bone marrow involvement was documented in 35 of the TRIL patients; a small-cell lymphoma was noted in 13, large cell in 15, both small and large cell in 4, and morphology was unavailable in 3 patients. All patients with DLBCL had 1 prior systemic therapy (n = 429); patients with TRIL had 1 (n = 52), 2 (n = 21), or 3 or more (n = 14) prior lines of systemic therapy. Prior therapy included rituximab in 68% of TRIL patients and 76% of DLBCL patients, with 36% of the TRIL patients and 23% of the DLBCL patients having received prior radiation. The proportion of patients with primary refractory disease (no response or progressive disease to anthracycline-based treatment) and early relapse (CR rate of <1 year) to last prior therapy was similar within the 2 groups (Table 1). Of the 87 TRIL patients, 43 received GDP and 44 received DHAP, whereas of the 429 DLBCL patients, 220 and 209 received GDP and DHAP, respectively.
Response rate, transplantation rate, and stem cell mobilization and maintenance
The response rate to salvage chemotherapy in TRIL patients was 47% and was also 45% in DLBCL patients (P = .81). Response rates appeared similar in patients receiving GDP or DHAP but were not statistically compared. The percentage of patients proceeding to ASCT (transplantation rate) was 52% in DLBCL and 53% in TRIL groups (P = 1.0). Successful PBSC mobilization (defined as ≥2 × 106 CD34+ cells/kg) was achieved in 84% of TRIL and 87% of DLBCL patients (Table 2).
Outcomes after chemotherapy and ASCT
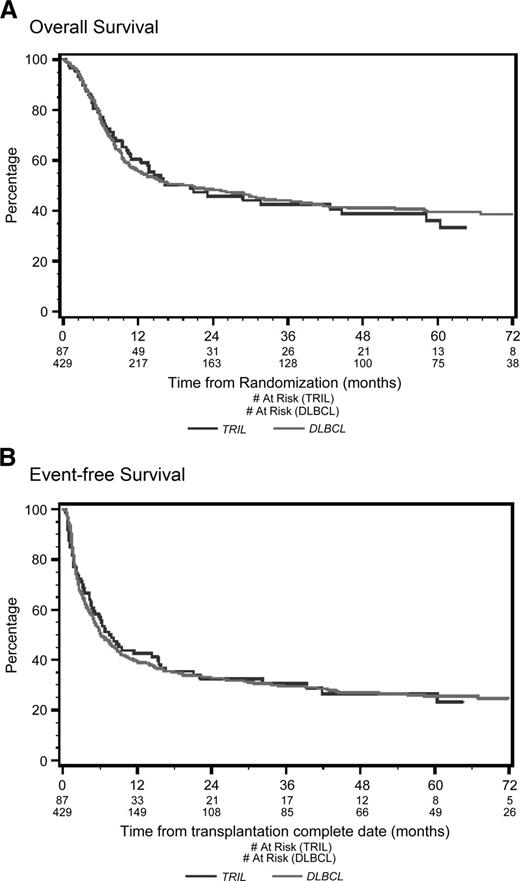
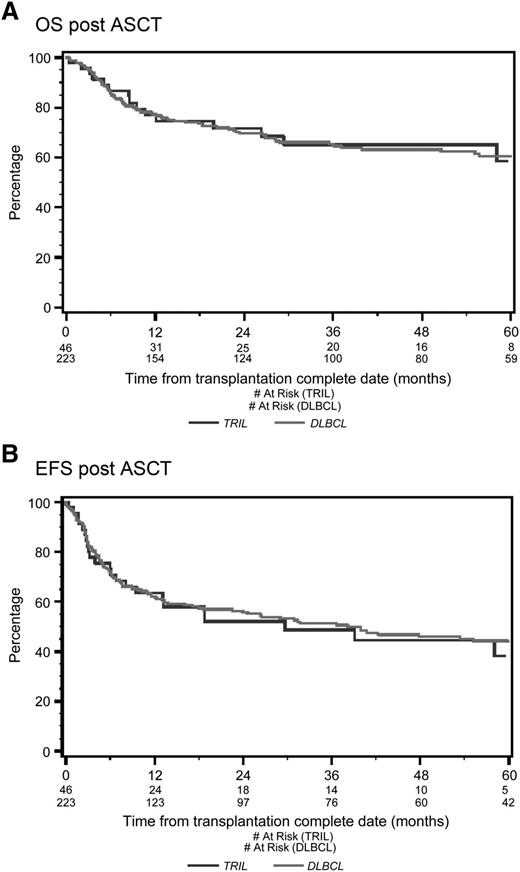
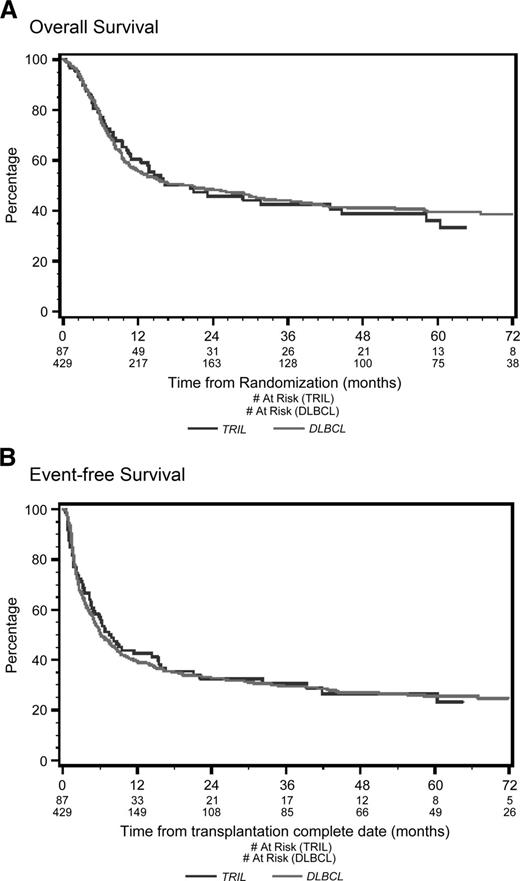
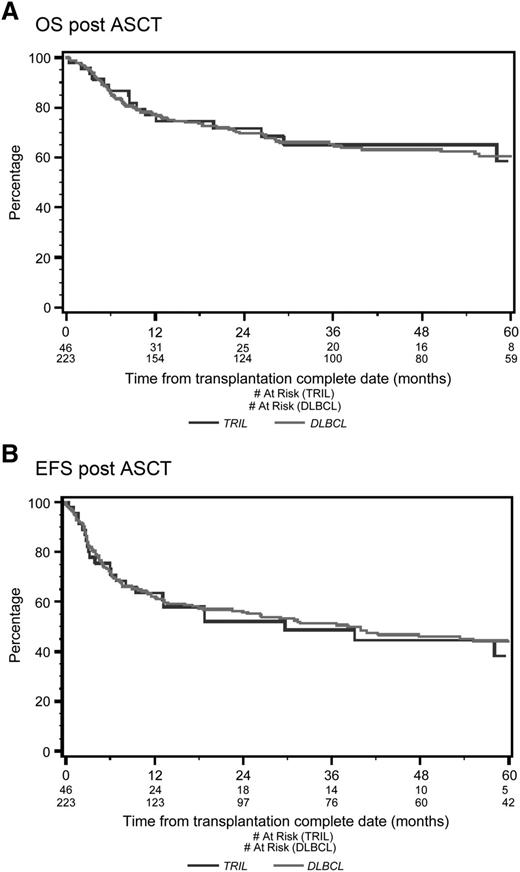
With a median follow-up of 53 months from the time of first randomization, the 4-year OS was 39% for TRIL patients and 41% for DLBCL patients (P = .78; Figure 1A). Four-year EFS was 27% for TRIL patients and 27% for DLBCL patients (P = .83; Figure 1B). Among patients who underwent ASCT, the 4-year OS was 65% for TRIL and 63% for DLBCL (P = .91; Figure 2A), whereas the EFS was 45% and 46%, respectively (P = .78; Figure 2B).
Overall survival and event-free survival from randomization. (A) Overall survival (OS) from randomization. With a median follow-up of 53 months, the 4-year OS was 48% for the TRIL cohort (blue line) and 51% for the DLBCL cohort (red line) (P = .61). (B) Event-free survival (EFS) from randomization. With a median follow-up of 53 months, the 4-year EFS was 26% for the TRIL cohort (blue line) and 27% for the DLBCL cohort (red line) (P = .61).
Overall survival and event-free survival from randomization. (A) Overall survival (OS) from randomization. With a median follow-up of 53 months, the 4-year OS was 48% for the TRIL cohort (blue line) and 51% for the DLBCL cohort (red line) (P = .61). (B) Event-free survival (EFS) from randomization. With a median follow-up of 53 months, the 4-year EFS was 26% for the TRIL cohort (blue line) and 27% for the DLBCL cohort (red line) (P = .61).
Overall survival and event-free survival from date of ASCT. (A) OS from date of ASCT. With a median follow-up of 53 months, the 4-year OS post-ASCT was 65% for the TRIL cohort (blue line) and 63% for the DLBCL cohort (red line) (P = .61). (B) EFS from date of ASCT. With a median follow-up of 53 months, the 4-year EFS was 45% for the TRIL cohort (blue line) and 46% for the DLBCL cohort (red line) (P = .97).
Overall survival and event-free survival from date of ASCT. (A) OS from date of ASCT. With a median follow-up of 53 months, the 4-year OS post-ASCT was 65% for the TRIL cohort (blue line) and 63% for the DLBCL cohort (red line) (P = .61). (B) EFS from date of ASCT. With a median follow-up of 53 months, the 4-year EFS was 45% for the TRIL cohort (blue line) and 46% for the DLBCL cohort (red line) (P = .97).
In total, 218 of the 516 patients were randomized to maintenance rituximab or observation. In the TRIL group, 16 received maintenance therapy and 22 were observed, whereas in the DLBCL group, 95 received rituximab and 85 were observed.
Adverse events during salvage therapy
Patients were evaluated using NCI Common Toxicity Criteria version 2.0. The rate of febrile neutropenia was 18% in the TRIL patients (11% in the GDP arm and 26% in the DHAP arm) and was 26% in the DLBCL patients (18% in the GDP arm and 35% in the DHAP arm). Hospitalizations for adverse events or illness occurred in 36% of TRIL patients (33% GDP, 40% DHAP) and 33% of DLBCL patients (25% GDP, 41% DHAP).
Prognostic factors and multivariate analysis
A multivariate Cox model was constructed to look at predictors of response, successful PBSC mobilization, transplantation rate, and OS and EFS. The multivariate results for OS and EFS are summarized in Table 3. Histology (TRIL or DLBCL) was not a predictor of any of these outcomes. Statistically significant adverse prognostic factors for ORR (data not presented), OS, and EFS were: refractory disease after primary therapy before study enrollment, lack of complete response/partial response to salvage therapy, and prior rituximab exposure. Additional significant adverse prognostic factors for OS and EFS were the presence of B symptoms or elevated lactate dehydrogenase (LDH).
Discussion
Although previous reports of patients with TRIL reported poor outcomes,10 more recent reports have suggested that survival may be improving: Recent data from the Iowa/Mayo SPORE described a median survival of 50 months post-transformation in their series.26 A series from the US National Comprehensive Cancer Network reported similar favorable outcomes with earlier follow-up.10 These improvements may be explained by earlier identification of transformed lymphoma and also by the impact of treatments such as rituximab-based chemotherapy and stem cell transplantation. Prospective or larger series of transplant-based strategies have not been reported until recently.13,15 Similarly, there have been limited data reporting the outcome of the denominator of patients being treated with a goal of ASCT despite the recognition that a significant proportion of patients will not undergo transplantation.11,16
This planned subset analysis of prospectively collected NCIC CTG LY.12 data represents the largest comparison of response to salvage therapy and outcomes post-ASCT for patients with transformed vs de novo DLBCL reported to date. Both patient groups were uniformly treated and evaluated, removing the selection bias inherent in cohort descriptions of single-institution series or those from transplant registries. Patients with TRIL who met eligibility criteria for LY.12 had a similar complete and overall response rate to salvage chemotherapy, and a similar likelihood of proceeding to ASCT as did those with DLBCL in the first relapse or progression. Although the follow-up is relatively short at 53 months for the entire cohort, 4-year OS and EFS were similar for the 2 groups. The aim of this analysis was to report the outcomes of relapsed/refractory TRIL using a clearly established standard of care for relapsed or refractory DLBCL (salvage chemotherapy and ASCT) to determine whether this treatment achieves similar results in TRIL.
Multivariate analysis of the population included in this analysis identified prior rituximab treatment, response to primary or salvage chemotherapy, elevated LDH, and B symptoms as being independent predictors of EFS; these 4 variables and the presence of multiple extranodal sites were also predictive of OS. Treatment arm (GDP or DHAP) and histology (TRIL or DLBCL) were not independently prognostic for EFS or OS.
The finding of prior rituximab-based therapy as an independent predictive factor in this analysis is similar to the results of the Coral trial in relapsed or refractory DLBCL and highlights the challenges of treating patients after they have progressive lymphoma after rituximab-based therapy.27 In relapsed and refractory DLBCL, the HOVON-44 trial reported improved response to salvage therapy and EFS with the addition of rituximab, compared with chemotherapy alone, but the large majority of patients in that trial had not been previously exposed to rituximab during primary therapy.28 The value of rituximab-based therapy for TRIL has been suggested in several available series, but the importance of including rituximab-retreatment during salvage therapy for TRIL before ASCT is not known.10,16,26
In the second randomization of LY12, patients with CD20+ lymphoma who had recovered from ASCT were assigned to rituximab 375 mg/m2 every 2 months for 6 doses, or observation.. Rituximab maintenance therapy after ASCT for follicular lymphoma has been recently reported to improve progression-free survival (PFS) but has not been evaluated prospectively in TRIL.29 It is possible that rituximab maintenance may be contributing to the favorable post-ASCT outcome of the TRIL cohort, but the small number of patients in this cohort may limit the interpretation of these data. The outcome data from the second randomization will be reported in a separate manuscript.
The total sample size of the NCIC CTG.LY12 cohort provided 80% power to detect clinically meaningful differences in overall response rate to salvage chemotherapy of 17% or higher (2-sided α = .05), but differences in this efficacy end point were not evident between the TRIL and DLBCL cohorts. Similarly, important adverse events such as febrile neutropenia and hospitalization for management of toxicity were also not statistically different. Given the potential for further additional chemotherapy exposure in the TRIL cohort as a result of the underlying indolent lymphoma, it is important to note that the efficacy in the TRIL patients was not associated with excessive toxicity. Although significant differences in efficacy or toxicity were not noted in this analysis of >500 patients, it remains a limitation of this dataset that the trial was not designed to study differences between these histologic subtypes.
In addition, biopsy at the time of relapse post-transplant was not mandated in the study protocol; therefore, it is not known in the patients with TRIL whether relapse was caused by the aggressive or indolent component of their disease. Transformation to DLBCL from an indolent lymphoma is accompanied by a complex series of molecular events,30,31 and biopsy of patients with TRIL at time of progression post-ASCT represents an opportunity to further understand the potential role of, for example, novel targeted therapies in the pre- and posttransplant setting.
Although there are few series that have reported the outcomes of patients with TRIL undergoing salvage chemotherapy and ASCT, our results compare favorably with these smaller and typically retrospective series. The major limitations of this analysis include the relatively modest sample size of 89 patients and lack of uniform exposure to rituximab in the TRIL cohort. Unfortunately, the other available series do not address these issues optimally. A prospective study from Norway evaluating chemotherapy and ASCT reported the outcome of 47 patients in which chemo-sensitive patients would undergo ASCT.11 The response rate to chemotherapy pre-transplant was 72%, and 64% of patients underwent ASCT. For the entire cohort, the 5-year OS was 43% and for the transplanted patients, the 5-year OS and PFS post-ASCT were 47% and 32%, respectively. Many of the patients were accrued to this trial before the availability of rituximab for treatment of both the indolent or transformed aggressive histology, and this trial did not complete full accrual.
Investigators at the Princess Margaret Hospital reported retrospective results of a cohort of TRIL patients treated with salvage chemotherapy and ASCT between1996 and 2009.16 One-hundred five patients with TRIL underwent chemotherapy with cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone (18%), a variety of platinum-containing regimens (63%), or rituximab-based therapy (27%). The response rate to chemotherapy was 57%, with 48% of all patients proceeding to ASCT. Three-year OS and PFS post-ASCT were 54% and 42%, respectively. Patients transplanted after 2004 or who received rituximab-based therapy immediately before ASCT had improved OS.
Many questions in the management of TRIL remain unanswered. However, we believe our analysis provides clarity in the management of patients with relapsed or refractory TRIL. The response to salvage chemotherapy, the ability to collect peripheral blood stem cells, and EFS and OS for patients with TRIL are similar to those for de novo DLBCL and support the use of salvage chemotherapy and ASCT as the standard of care for this patient population.
Presented in part at the 12th International Conference on Malignant Lymphoma June 19-22, 2013, Lugano, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the research and clinical personnel who spent many years looking after patients to complete this study.
The National Cancer Institute of Canada Clinical Trials Group is supported by the Canadian Cancer Society Research Institute. This study was supported by Lilly Canada and Roche Canada.
Authorship
Contribution: J.K., A.E.H., L.S., B.E.C., and M.Crump conceived and designed the study; all authors provided study materials or patients, collected and assembled data, and interpreted and analyzed data; J.K., A.E.H., L.S., B.E.C., and M.Crump wrote the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: J.K. received honoraria, consultancy, and research funding from Roche Canada. D.A.M. and C.T.K. received research funding from Roche Canada. H.J.O. is a consultant to Roche Canada. P.A. received honoraria from Roche Canada. S.L. received research funding from Roche. M.Crump received honoraria and research funding from Roche Canada.
Correspondence: John Kuruvilla, Princess Margaret Cancer Centre, Rm 5-110, 610 University Ave, Toronto, ONT, Canada, M5G 2M9; e-mail: john.kuruvilla@uhn.ca.