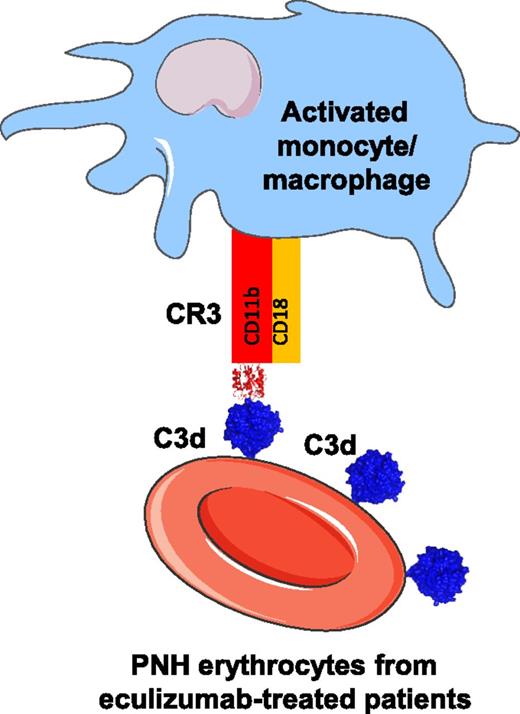
In this issue of Blood, Lin et al elegantly demonstrate that the erythrocytes from patients with paroxysmal nocturnal hemoglobinuria (PNH) undergoing eculizumab treatment, which are opsonized with the complement C3dg, can interact with complement receptor 3 (CR3) on activated monocytes, thus leading to erythrophagocytosis.1
Mechanism of C3dg-CR3–mediated erythrophagocytosis. The erythrocytes of patients with PNH and treated with eculizumab are opsonized by C3dg. C3dg serves as a ligand of CR3, facilitating the erythrophagocytosis by activated monocytes and potentially by macrophages. The cell shapes in the figure are taken from Servier Medical Art database (http://www.servier.fr/smart/banque-dimages-powerpoint). The atomic coordinates of the integrin I domain of the CR3-C3d complex (Protein Data Bank ID code 4M76) and the C3d domain taken out of it are used for the representation.
Mechanism of C3dg-CR3–mediated erythrophagocytosis. The erythrocytes of patients with PNH and treated with eculizumab are opsonized by C3dg. C3dg serves as a ligand of CR3, facilitating the erythrophagocytosis by activated monocytes and potentially by macrophages. The cell shapes in the figure are taken from Servier Medical Art database (http://www.servier.fr/smart/banque-dimages-powerpoint). The atomic coordinates of the integrin I domain of the CR3-C3d complex (Protein Data Bank ID code 4M76) and the C3d domain taken out of it are used for the representation.
The central complement system component C3 undergoes a complex cascade of activation steps, generating multiple activation fragments (C3a, C3b, iC3b, C3dg, C3d, etc), each of which interacts with different receptors and has distinct functions.2,3 After the discovery of the complement receptors ∼35 years ago, CR3 was considered essential for phagocytosis of iC3b opsonized immune complexes and pathogens, without interacting with C3dg.4 Nevertheless, recent studies have demonstrated that CR3 interacts with C3dg.5 Lin et al provide evidence for the pathophysiologic relevance of this interaction in the context of the PNH. In this disease, the red blood cells of patients lack 2 membrane-expressed complement regulators, CD55 and CD59, and hence are susceptible to complement-mediated lysis, leading to a life-threatening intravascular hemolysis. The approval for clinical use of the complement C5- blocking antibody eculizumab revolutionized the treatment of these patients, because it prevents complement-mediated intravascular hemolysis.6 It is noteworthy that eculizumab blocks only the late stages of the complement cascade, thus leading to an accumulation of C3d(g)-opsonized erythrocytes in the circulation of patients with PNH.
Lin et al investigate the role of C3dg opsonization for the phagocytosis of these cells. They confirm that C3dg can interact with the phagocytic receptor CR3 using purified proteins. Further, they demonstrate that this interaction can occur at the phagocytic synapse between patients’ erythrocytes and activated monocytes (see figure). The level of erythrophagocytosis was linearly correlated with the level of C3d opsonization of patients’ purified erythrocytes. These results provide a hint to explain the residual hemolysis that occurs despite treatment with eculizumab in some PNH patients. Further studies are needed, however, to identify the proportion of cells that are lysed by the proposed mechanism in vivo. A recent study correlated the level of hemolysis in PNH patients with the level of free eculizumab present in the circulation.7 They found that the low levels of circulating eculizumab (measured at the moment before the next injection of the drug) correlated with a detectable complement activity, the presence of hemolysis, and a need for blood transfusion. Moreover, in this study, the level of C3d(g) on patients’ erythrocytes did not correlate with increased hemolysis or a need for blood transfusion. Analysis in 2 additional cohorts (where the level of free eculizumab was not measured) suggested that the opsonization of PNH erythrocytes by C3d(g) leads to extravascular clearance of these cells, and that this type of clearance may contribute to the low level of hemolysis and residual transfusion requirement observed in some patients on eculizumab therapy.8,9 Therefore, larger studies are needed to clarify the exact extent of the C3d(g)-mediated extravascular hemolysis in PNH patients under eculizumab treatment.
The results of Lin et al presented here describe a novel mechanism of erythrophagocytosis based on the CR3-C3d interaction, which may be operational in pathophysiologic conditions. If occurring in vivo, this mechanism provides a rationale for optimization of the therapy in some patients, who remain transfusion dependent despite the eculizumab treatment, using complement inhibitors acting on the level of C3. This phenomenon needs attention, because it may not be restricted to PNH but may also be relevant to other diseases, characterized by C3d deposition on cells, including autoimmune hemolytic anemia, graft rejection, and atherosclerosis.
Conflict-of-interest disclosure: The author declares no competing financial interests.