In this issue of Blood, Ceriani et al introduce, in primary mediastinal B-cell lymphoma (PMBCL), a new prognostic factor measured on pretreatment 18F-fluorodeoxyglucose (18FDG)-positron emission tomography (PET)/computed tomography (CT): the total lesion glycolysis (TLG), which is an index of the glucose uptake by the total tumor burden.1 This paper is part of the International Extranodal Lymphoma Study Group (IELSG) 26 prospective study designed to evaluate the role of PET in the treatment of PMBCL.2
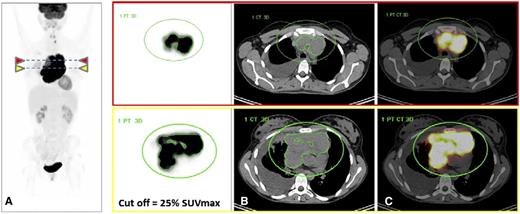
FDG-PET/CT images from a 25-year-old woman with PMBCL who progressed and died under treatment with R-MACOP-B. (A) FDG-PET images (maximum intensity projection view and transverse slices at 2 different levels) demonstrate high FDG uptake in a bulky mediastinal mass; the green circle defines a volume of interest where the MTV is automatically segmented with a 25% SUVmax threshold. MTV limits are displayed in green around the mediastinal tumor. SUVmax = 29; TLG = 6706, falling in the high-risk category. (B) CT with the projections of the MTV contours segmented on FDG-PET. (C) Fused FDG-PET and CT images. Courtesy of Dr Luca Ceriani.
FDG-PET/CT images from a 25-year-old woman with PMBCL who progressed and died under treatment with R-MACOP-B. (A) FDG-PET images (maximum intensity projection view and transverse slices at 2 different levels) demonstrate high FDG uptake in a bulky mediastinal mass; the green circle defines a volume of interest where the MTV is automatically segmented with a 25% SUVmax threshold. MTV limits are displayed in green around the mediastinal tumor. SUVmax = 29; TLG = 6706, falling in the high-risk category. (B) CT with the projections of the MTV contours segmented on FDG-PET. (C) Fused FDG-PET and CT images. Courtesy of Dr Luca Ceriani.
PMBCL is a clinicopathologic entity of aggressive B-cell lymphoma that is clinically and biologically distinct from the other molecular subtypes of diffuse large B-cell lymphoma (DLBCL) and accounts for 2% to 4% of all non-Hodgkin lymphomas. It carries a relatively favorable prognosis in comparison with DLBCL as a result of patients’ younger age and earlier stage at presentation.3 A 5-year survival rate >90% is reported in recent studies under aggressive immunochemotherapy with or without consolidation radiotherapy. Successful primary treatment is critical for PMBCL management because patients who failed or relapsed had a dismal outcome. A subset of patients develops an early refractory disease often around the completion of the first course of treatment, with a high failure rate after salvage treatment. Therefore, there is a need to identify these high-risk patients early when alternative therapeutic strategy, including more intensive treatment, could be considered. Prognosis factors are warranted for tailoring therapy and sparing the majority of young patients from the toxicity of these regimens. The international prognostic index (IPI) or age-adjusted IPI has not proven adequate. Other proposed clinical predictors of survival have not been validated prospectively, and biomarkers useful for risk stratification are now unavailable.3,4
18FDG is an imaging biomarker surrogate of glucose metabolism.5 The degree of FDG uptake observed in lymphoma subtypes depends on the uptake of both tumor cells and cells from the microenvironment. It is impacted by the cross talk between them. In Hodgkin lymphoma (HL), FDG is chiefly a biomarker of the recruited accessory cells that account for >95% of the tumor, whereas in DLBCL, the uptake is mainly due to lymphoma cells.5 PMBCL is an FDG-avid lymphoma sharing some molecular characteristics with these 2 entities. The uptake is likely due to the lymphoma cells that are the main component of the tumor, but an uptake by the microenvironment cannot be excluded, as recent data have highlighted its important role in PMBCL.4
18FDG-PET has been recognized as the best imaging tool for staging and response assessment in FDG-PET-avid lymphoma.6 Recently, Martelli et al reported (within the IELSG 26 study) a high prognostic value for end-of-treatment PET (eot-PET) in 125 PMBCL patients.2 The negative predictive value (NPV) was 98% for progression-free survival (PFS), which suggested that, on the basis of negative eot-PET, it could be possible to safely reduce the number of PMBCL patients to whom radiotherapy is given.
However, although the prognostic values of interim or eot-PET have been extensively studied in lymphoma, the role of quantitative data derived from baseline PET has been investigated less due to technical limitations. In HL, DLBCL, and T-cell lymphoma, prognostic values of baseline metabolic tumor volume (MTV) and TLG have been reported only on the basis of retrospective series.5 Ceriani et al report on the first study prospectively exploring the value of quantitative PET in aggressive lymphoma, focusing on 103 PMBCL patients. Maximum standardized uptake value (SUVmax), MTV, and TLG, all parameters dependent on lymphoma metabolism, measured on baseline FDG-PET/CT were negative prognostic factors for PFS and overall survival (OS). A higher risk of progression or deaths was associated with an MTV and TLG increase, but TLG was the strongest predictor of outcome independent of the stage of the disease. Long-term outcome was significantly better for patients with low TLG (less than the cutoff value) than for those with high TLG (greater than the cutoff value) with a 5-year OS of 100% vs 80% and 5-year PFS of 99% vs 64%, respectively. The method based on a percent thresholding of the tumor SUVmax was easy to use and reproducible (see figure). The technical difficulties of quantitative PET were minimized in PMBCL because tumor burden is mainly limited to a single bulky mass, which gives strength to the results of this study.
The high glycolytic activity observed in the high-risk patients might be explained by the increased proliferation of the malignant cells induced by the dysregulation of major signaling pathways and/or by the functional consequences for the microenvironment of their genetic alterations.4,7,8
Although Ceriani et al report very high sensitivity (92%) for TLG with a 98% NPV for PFS, the positive predictive value (PPV) was only 36%. Quantitative PET accurately identifies low-risk patients, but a better selection of high-risk patients is warranted to submit them to intensive treatment, and further studies are needed. To increase the risk stratification obtained with quantitative PET, it has been proposed to combine the PET baseline data with the PET response data or other clinical/biologic parameters, a method called integrative PET. In HL, a combination of baseline MTV with interim PET (iPET) has improved risk stratification, and iPET-negative patients could be stratified according to different risk molecular profiles.5 In this regard, Zucca et al9 reported in the same series of PMBCL patients that the combination of baseline TLG with end treatment PET more accurately identified patients at risk, with a PPV reaching 47% without a detrimental effect on NPV.
Because early stratification is preferred before the end of first-line therapy, other approaches could be investigated to define new prognostic models: baseline PET data can be combined with other clinical data or with molecular data such as the presence of XPO1 mutations recently reported as a recurrent alteration, which could be a biomarker of prognostic impact10 ; they can also be combined with other PET data such as the heterogeneity of the SUV distribution in the tumor or with parameters obtained from other imaging techniques such as diffusion-weighted magnetic resonance imaging (MRI).
This stimulating study has opened an exciting field. It might be possible to use baseline quantitative FDG-PET to provide an earlier definition of a risk-adapted therapeutic strategy in PMBCL with this new imaging biomarker of tumor metabolism.
Conflict-of-interest disclosure: The author declares no competing financial interests.