Key Points
18FDG PET/CT is a very important staging tool for patients with PMBCL.
Metabolic activity defined by TLG on the baseline PET scan is a powerful predictor of PMBCL outcome.
Abstract
The International Extranodal Lymphoma Study Group (IELSG) 26 study was designed to evaluate the role of 18F-fluorodeoxyglucose (18FDG) positron emission tomography/computed tomography (PET/CT) in the management of primary mediastinal (thymic) large B-cell lymphoma (PMBCL). We examined the prognostic impact of functional PET parameters at diagnosis. Metabolic activity defined by the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) was measured on baseline 18FDG PET/CT following a standard protocol in a prospectively enrolled cohort of 103 PMBCL patients. All received combination chemoimmunotherapy with doxorubicin- and rituximab-based regimens; 93 had consolidation radiotherapy. Cutoff values were determined using the receiver-operating characteristic curve. At a median follow-up of 36 months, progression-free survival (PFS) and overall survival (OS) were 87% and 94%, respectively. In univariate analysis, elevated MTV and TLG were significantly associated with worse PFS and OS. Only TLG retained statistical significance for both OS (P = .001) and PFS (P < .001) in multivariate analysis. At 5 years, OS was 100% for patients with low TLG vs 80% for those with high TLG (P = .0001), whereas PFS was 99% vs 64%, respectively (P < .0001). TLG on baseline PET appeared to be a powerful predictor of PMBCL outcomes and warrants further validation as a biomarker. The IELSG 26 study was registered at www.clinicaltrials.gov as #NCT00944567.
Introduction
Primary mediastinal (thymic) large B-cell lymphoma (PMBCL) is a distinct clinicopathological1-3 and molecular4,5 subtype of diffuse large B-cell lymphoma (DLBCL) arising from a small population of B cells within the thymus. It is characterized by a rapidly progressive anterior mediastinal mass, often with local invasion and compressive syndromes, and by recurrence at unusual extranodal sites.6 PMBCL commonly affects young adults, and treatment with aggressive chemotherapy regimens and rituximab with or without radiotherapy appears to yield better outcomes than in other DLBCLs, partly as a result of the younger age and earlier stage at presentation, with 5-year survival rates of over 90% in recent studies.7,8
However, salvage treatment of the few patients failed by initial therapy has a poor outcome, as a result of rapidly emerging chemorefractory disease, often around the completion of the first course of treatment.9,10 If it were possible to predict this important minority, it would allow for the development of risk-stratified approaches such as early intensification with infusional regimens or even elective myeloablative therapy.
Hence, there is a need for reliable prognostic markers. The International Prognostic Index (IPI), the conventional tool used to predict outcome for DLBCL, has limited utility in PMBCL because of the young patient age and the usual confinement of the disease to the mediastinum.11 This is reflected in the observation that most patients have low-risk IPI scores at presentation.8,12
18F-fluorodeoxyglucose (18FDG) positron emission tomography/computed tomography (PET/CT) is increasingly used to monitor treatment response and guide clinical decisions on treatment strategies for FDG-avid lymphomas, including PMBCL.13-15 We have previously shown8 that liver FDG uptake was the optimal cutoff value to predict outcome, providing a validation of the utility for a 5-point scale (Deauville criteria, initially developed for the interim PET evaluation of Hodgkin disease and DLBCL)16 in end-of-therapy PET evaluation of PMBCL.
The objective of the present study was to investigate whether the main quantitative baseline PET-derived metabolic parameters can predict prognosis, and to compare these parameters with the commonly used clinical indices, in patients with PMBCL.
Patients and methods
Patient population
Between January 2007 and July 2010, 125 patients with histologically proven PMBCL were prospectively enrolled in the International Extranodal Lymphoma Study Group (IELSG) 26 study (http://clinicaltrials.gov/ct2/show/NCT00944567) and treated with the R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP-like R-VACOP-B (rituximab plus etoposide, leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) or R-MACOP-B (rituximab plus methotrexate, leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) regimens according to local policy. Consolidation with mediastinal involved field radiotherapy as indicated by local guidelines was allowed; all patients treated received, within 8 weeks of the last dose of chemotherapy, a dose of at least 30 Gy on the original tumor volume. Treatment details have been reported previously.8 The study was conducted in accordance with the precepts of the Helsinki declaration and received approval from the local research ethical committee of each participating center.
18FDG-PET imaging procedures
The study protocol included mandatory technical procedures for the acquisition and elaboration of PET imaging, based on the state of the art at the time when the study was planned.17 Baseline PET scans were performed within 14 days before the first chemotherapy. In cases where urgent treatment was required and the PET scan could not be performed before therapy started, the baseline scan was omitted after discussion with the clinical coordinators; these cases were excluded from this analysis. PET/CT imaging was performed on full-ring integrated PET/CT systems. Each center was required to follow an active quality-control and quality-assessment protocol.17 PET and CT images were acquired in the same session. CT scans obtained with a low-dose protocol were used for attenuation-correction of the PET images. All patients were fasting for at least 6 hours before the injection of 250 to 370 MBq (4.5 MBq/kg) 18FDG. Blood glucose measured before injection of the radiotracer was <160 mg/dL in all patients. PET data were acquired in 2- or 3-dimensional mode from the midthigh toward the base of the skull after a standardized uptake time of 60 minutes (±5 minutes). The PET acquisition time was at least 3 minutes per bed position. Images were reconstructed with validated and commercially available iterative algorithms, and standardized uptake values (SUV) were automatically calculated. All PET/CT image data were stored in Digital Imaging and Communications in Medicine format and sent to the core laboratory for central review. The adherence to the technical protocol requirements was centrally controlled for each data set. A central blind analysis was performed by one trained nuclear physician (L.C.) without information on the clinical outcome.
18FDG PET parameters
The 18FDG-PET/CT images were obtained at baseline for initial staging in 103 out of 125 patients and analyzed following a standard protocol on a dedicated workstation (Siemens Syngo MMWP Workstation VE36A; Siemens, Erlangen, Germany). Dedicated software (Syngo TrueD) automatically estimated the average and maximum SUV (SUVmean and SUVmax) and metabolic tumor volume (MTV) of the entire mediastinal lesion using an isocontour threshold method based on 25% of the SUVmax. In 8 cases, the boundaries of the lesion estimated automatically were partly manually redrawn to exclude physiological cardiac activity . The total lesion glycolysis (TLG) was then calculated as the product of SUVmean and MTV.18
After an experimental evaluation of different threshold levels (20%, 25%, 30%, 35%, 41%, and 50% of the SUVmax) carried out on a subgroup of 20 cases, 25% of the SUVmax was identified as the best cutoff value. The choice of the threshold was based on visual comparative analysis performed by 2 independent observers (L.C. and L.G.), taking into account the best fit between “metabolic edges” and anatomical boundaries, in particular at the interface between tumor lesion and lung tissue, which is the most clearly identifiable on the CT low-dose images. The presence of extramediastinal disease (found in 14/103 baseline PET scans) contributed significantly to the MTV in only 1 patient (29% of the total volume), whereas in all the others, the lesions were very small (usually single FDG-positive distant lymph nodes), involving <5% of the MTV value. This methodology showed very high interobserver reproducibility (Pearson correlation coefficient >0.99 and P < .0001) for the calculation of all quantitative parameters.
Statistical methods
The PET-associated functional parameters were analyzed as both continuous and dichotomized variables, using receiver-operating characteristic (ROC) analysis to identify the optimal cutoff point. Overall survival (OS) and progression-free survival (PFS) were defined according to the revised National Cancer Institute criteria13 and estimated using the Kaplan-Meier or life-table method as appropriate. Follow-up was calculated as the median time to censoring using a reverse Kaplan-Meier analysis.19 Differences between survival curves associated with discrete or dichotomized variables were analyzed using the log-rank test. Cox regression was used to estimate the hazard ratio (HR) and its confidence interval (CI). The exact 95% CIs were calculated for incidence percentages. P values of ≤ .05 (2-sided test) were considered to indicate statistical significance. Statistical analysis was conducted using the STATA 11 software package (Stata Corp, College Station, TX).
Results
Detailed clinical features and outcome of the patients enrolled in the IELSG 26 study have been published previously.8 Baseline PET/CT studies were available in 103 out of 125 patients, having been omitted in 20 patients because of the urgency of treatment and excluded in 2 cases (after central control) on technical grounds due to unacceptable lengthening of the uptake time. Table 1 summarizes the demographic and clinical characteristics of the 103 patients included in the present analysis. All had initial treatment with rituximab- and anthracycline-containing regimens, and 93 also had consolidation radiotherapy to the mediastinum. At a median follow-up of 36 months (interquartile range, 31-45 months), 13 patients had disease progression or relapse and 6 had died. The estimated 5-year PFS and OS rates were 87% (95% CI, 79%-92%) and 94% (95% CI, 87%-97%), respectively.
Patient clinical characteristics at presentation
| Characteristic (n = 103 unless otherwise specified) . | No. (%) of patients . |
|---|---|
| Age | |
| Median (interquartile range), y | 33 (26-41) |
| ≤60 y | 97 (94) |
| Female sex | 63 (61) |
| Performance status | |
| ECOG 0-1 | 88 (85) |
| ECOG >1 | 15 (15) |
| B-symptoms at presentation | 38 (37) |
| Bulky disease | |
| Max mediastinal lesion >7 cm | 89 (86) |
| Max mediastinal lesion >10 cm | 54 (52) |
| Extramediastinal involvement ≥1 site | 14 (14) |
| Bone marrow involvement | 2 (2) |
| Ann Arbor stage | |
| I-II | 97 (94) |
| III-IV | 6 (6) |
| LDH >normal upper value* | 77 (75) |
| IPI | |
| Low and low-intermediate risk | 99 (96) |
| Intermediate-high and high risk | 4 (4) |
| aaIPI (n = 97) | |
| Low and low-intermediate risk | 82 (85) |
| Intermediate-high and high risk | 15 (15) |
| Front-line treatment | |
| R-CHOP or R-CHOP like regimen* | 16 (16) |
| R-VACOP-B or R-MACOP-B regimen* | 87 (84) |
| RT | 93 (90) |
| Characteristic (n = 103 unless otherwise specified) . | No. (%) of patients . |
|---|---|
| Age | |
| Median (interquartile range), y | 33 (26-41) |
| ≤60 y | 97 (94) |
| Female sex | 63 (61) |
| Performance status | |
| ECOG 0-1 | 88 (85) |
| ECOG >1 | 15 (15) |
| B-symptoms at presentation | 38 (37) |
| Bulky disease | |
| Max mediastinal lesion >7 cm | 89 (86) |
| Max mediastinal lesion >10 cm | 54 (52) |
| Extramediastinal involvement ≥1 site | 14 (14) |
| Bone marrow involvement | 2 (2) |
| Ann Arbor stage | |
| I-II | 97 (94) |
| III-IV | 6 (6) |
| LDH >normal upper value* | 77 (75) |
| IPI | |
| Low and low-intermediate risk | 99 (96) |
| Intermediate-high and high risk | 4 (4) |
| aaIPI (n = 97) | |
| Low and low-intermediate risk | 82 (85) |
| Intermediate-high and high risk | 15 (15) |
| Front-line treatment | |
| R-CHOP or R-CHOP like regimen* | 16 (16) |
| R-VACOP-B or R-MACOP-B regimen* | 87 (84) |
| RT | 93 (90) |
RT, consolidation mediastinal radiotherapy.
Details on the immunochemotherapy regimens and radiotherapy plans have been previously reported.8
The description of the baseline functional PET parameters studied, SUVmax, MTV, and TLG is depicted in Table 2, which summarizes the results of the ROC analysis used to identify optimal cutoff points.
Baseline 18FDG-PET parameters
| Parameter . | Median . | Interquartile range . | ROC curve for PFS . | ROC curve for OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) . | P . | Cutoff . | Sensitivity (95% CI) . | Specificity (95% CI) . | AUC (95% CI) . | P . | Cutoff . | Sensitivity (95% CI) . | Specificity (95% CI) . | |||
| SUVmax | 18.8 | 15.5-23 | 0.647 (0.476-0.819) | .09 | 22.2 | 61.5% (31.6-86.1) | 75.6% (65.4-84.0) | 0.711 (0.488-0.935) | .06 | 22.2 | 83.3% (35.9-99.6) | 74.2% (64.3-92.6) |
| MTV | 406 | 267-641 | 0.814 (0.681 -0.946) | .0001 | 703 | 69% (38.6-90.9) | 87.8% (79.2-93.7) | 0.812 (0.661-0.941) | .0001 | 490 | 100% (54.1-100) | 60.8% (50.4-70.6) |
| TLG | 4261 | 2363-6398 | 0.867 (0.746-1.0) | .0001 | 5814 | 92.1% (64.0-99.8) | 77.7% (66.6-84.9) | 0.921 (0.841-1.0) | .0001 | 6031 | 100% (54.1-100) | 74.2% (64.3-82.6) |
| Parameter . | Median . | Interquartile range . | ROC curve for PFS . | ROC curve for OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) . | P . | Cutoff . | Sensitivity (95% CI) . | Specificity (95% CI) . | AUC (95% CI) . | P . | Cutoff . | Sensitivity (95% CI) . | Specificity (95% CI) . | |||
| SUVmax | 18.8 | 15.5-23 | 0.647 (0.476-0.819) | .09 | 22.2 | 61.5% (31.6-86.1) | 75.6% (65.4-84.0) | 0.711 (0.488-0.935) | .06 | 22.2 | 83.3% (35.9-99.6) | 74.2% (64.3-92.6) |
| MTV | 406 | 267-641 | 0.814 (0.681 -0.946) | .0001 | 703 | 69% (38.6-90.9) | 87.8% (79.2-93.7) | 0.812 (0.661-0.941) | .0001 | 490 | 100% (54.1-100) | 60.8% (50.4-70.6) |
| TLG | 4261 | 2363-6398 | 0.867 (0.746-1.0) | .0001 | 5814 | 92.1% (64.0-99.8) | 77.7% (66.6-84.9) | 0.921 (0.841-1.0) | .0001 | 6031 | 100% (54.1-100) | 74.2% (64.3-82.6) |
In univariate analysis of the PET parameters as continuous variables (Cox regression), a significantly higher risk of progression was associated with an increase in MTV (HR, 1.36; 95% CI, 1.16-1.61 for any increment of 102 mL, P < .001) and TLG (HR, 1.40; 95% CI, 1.25-1.58 for any increment of 103 mL; P < .001), but not with an increase in SUVmax (HR, 1.08; 95% CI, 0.98-1.18 for any increment of 1 unit; P = .119). OS also appeared to be significantly correlated with an increase in either MTV (HR, 1.39 for each increment of 102 mL; 95% CI, 1.08-1.77; P = .009) or TLG (HR, 1.47 for each 103-unit increment; 95% CI, 1.22-1.76; P < .001), but not SUVmax (HR, 1.11;95% CI, 0.97-1.27 for any increment of 1 unit, P = .117).
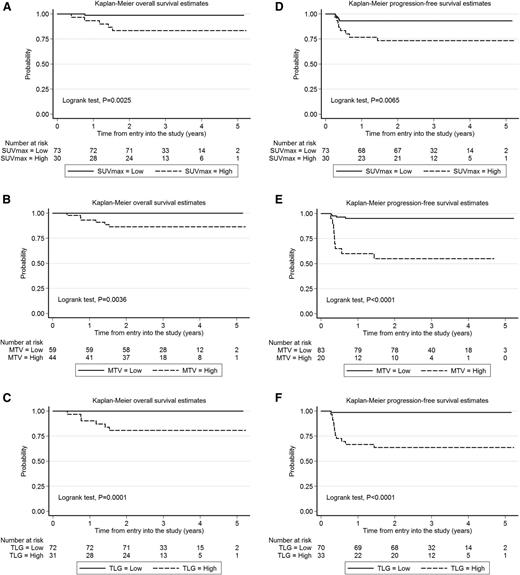
Univariate analysis of dichotomized parameters (log-rank test) showed a statistically significant association of high SUVmax, MTV, and TLG with worse PFS and OS (Figure 1). Elevated TLG and MTV were more frequently seen in patients with bulk >10 cm (supplemental Table 1, available on the Blood Web site). Among the clinical features at presentation, bulk >10 cm was also associated with shorter PFS, but not OS (Table 3), whereas no impact on outcome was seen in this study population for gender, age, performance status, B-symptoms, serum lactate dehydrogenase (LDH), stage, IPI, and age-adjusted IPI (aaIPI) (supplemental Table 2). Nevertheless, on multivariate analysis (Table 4), only TLG retained statistical significance for both OS and PFS controlling for MTV (Cox models for either PFS or OS) and tumor bulk (included only in the Cox model for PFS).
Estimates of OS and PFS according to the value of functional 18FDG-PET parameters in the PET/CT scan performed during the initial staging of 103 patients with primary mediastinal large cell lymphoma enrolled in the IELSG 26 prospective study. (A-C) Kaplan-Meier estimates of OS. (D-F) Kaplan-Meier estimates of PFS. Cutoff values are reported in Table 2. High, above the cutoff values calculated by ROC analysis; low, at or below the cutoff values calculated by ROC analysis.
Estimates of OS and PFS according to the value of functional 18FDG-PET parameters in the PET/CT scan performed during the initial staging of 103 patients with primary mediastinal large cell lymphoma enrolled in the IELSG 26 prospective study. (A-C) Kaplan-Meier estimates of OS. (D-F) Kaplan-Meier estimates of PFS. Cutoff values are reported in Table 2. High, above the cutoff values calculated by ROC analysis; low, at or below the cutoff values calculated by ROC analysis.
Univariate analysis (life tables and log-rank test) of the difference between OS and PFS according to dichotomized baseline PET parameters and the presence of a bulky lesion
| Parameter . | 5-y OS (95% CI) . | P . | 5-y PFS (95% CI) . | P . |
|---|---|---|---|---|
| SUVmax | .0025 | .0065 | ||
| Low | 99% (91%-99.8%) | 93% (84%-97%) | ||
| High | 83% (64%-93%) | 73% (54%-86%) | ||
| MTV | .0036 | <.0001 | ||
| Low | 100% (n.a.%) | 95% (88%-98%) | ||
| High | 86% (72%-94%) | 55% (31%-73%) | ||
| TLG | .0001 | <.0001 | ||
| Low | 100% (n.a.%) | 99% (90%-99.8%) | ||
| High | 80% (62%-91%) | 64% (45%-77%) | ||
| Bulky disease | .120 | .013 | ||
| <10 cm | 98% (86%-99%) | 96% (85%-99%) | ||
| ≥10 cm | 91% (79%-96%) | 80% (66%-88%) |
| Parameter . | 5-y OS (95% CI) . | P . | 5-y PFS (95% CI) . | P . |
|---|---|---|---|---|
| SUVmax | .0025 | .0065 | ||
| Low | 99% (91%-99.8%) | 93% (84%-97%) | ||
| High | 83% (64%-93%) | 73% (54%-86%) | ||
| MTV | .0036 | <.0001 | ||
| Low | 100% (n.a.%) | 95% (88%-98%) | ||
| High | 86% (72%-94%) | 55% (31%-73%) | ||
| TLG | .0001 | <.0001 | ||
| Low | 100% (n.a.%) | 99% (90%-99.8%) | ||
| High | 80% (62%-91%) | 64% (45%-77%) | ||
| Bulky disease | .120 | .013 | ||
| <10 cm | 98% (86%-99%) | 96% (85%-99%) | ||
| ≥10 cm | 91% (79%-96%) | 80% (66%-88%) |
The presence of a bulky lesion was statistically significant only for PFS. All the remaining baseline clinical parameters, namely, gender, age, performance status, B-symptoms, LDH, stage, and the international prognostic indices, had no statistically significant impact on both OS and PFS and are not included in this table but are reported in the online appendices. Cutoff values used for the dichotomization of functional PET parameters (SUVmax, MTV, TLG) are reported in Table 2.
Multivariate analysis (Cox proportional hazard models for either OS or PFS)
| Parameter . | HR . | 95% CI . | P . |
|---|---|---|---|
| Multivariate analysis of OS (103 subjects, 6 deaths) | |||
| MTV (increments of 102 ml) | 0.96 | 0.66-1.40 | .833 |
| TLG (increments of 103) | 1.49 | 1.18-1.89 | .001 |
| Multivariate analysis of PFS (103 subjects, 13 events) | |||
| Bulky disease (<10 cm vs ≥10 cm) | 1.73 | 0.31-9.52 | .526 |
| MTV (increments of 102 mL) | 1.03 | 0.80-1.33 | .812 |
| TLG (increments of 103 mL) | 1.36 | 1.16-1.58 | <.001 |
| Parameter . | HR . | 95% CI . | P . |
|---|---|---|---|
| Multivariate analysis of OS (103 subjects, 6 deaths) | |||
| MTV (increments of 102 ml) | 0.96 | 0.66-1.40 | .833 |
| TLG (increments of 103) | 1.49 | 1.18-1.89 | .001 |
| Multivariate analysis of PFS (103 subjects, 13 events) | |||
| Bulky disease (<10 cm vs ≥10 cm) | 1.73 | 0.31-9.52 | .526 |
| MTV (increments of 102 mL) | 1.03 | 0.80-1.33 | .812 |
| TLG (increments of 103 mL) | 1.36 | 1.16-1.58 | <.001 |
Each as a whole was statistically significant, and in both models, TLG resulted in a significantly longer survival time after controlling for mediastinal bulk and MTV in PFS and for MTV in OS.
Long-term outcome was significantly better for patients with low TLG (below the cutoff value) compared with those with high TLG (above the cutoff value), with 5-year OS of 100% vs 80% and 5-year PFS of 99% vs 64%, respectively (Figure 1).
Despite limited statistical power, baseline TLG remained the strongest predictor of outcome for the 82 patients with stage I or II disease in univariate analysis of OS (log-rank test, P = .0027) and PFS (log-rank test, P = .0001), as well as in Cox models controlling for MTV (HR, 4.21 with P = .018 for OS; HR, 1.70 with P = .009 for PFS).
Discussion
There is increasing evidence of the prognostic value of quantitative parameters obtained from initial staging by 18FDG-PET/CT in patients with high-grade non-Hodgkin lymphoma, in addition to the standard qualitative visual analysis.15,20,21 Initially, SUV was the most widely studied parameter.22-24 More recently, growing recognition of volume-based metabolic assessment has emerged, including MTV and TLG as promising prognostic indices in solid tumors,25 multiple myeloma,26 and malignant lymphomas.27-30 Among these functional parameters, TLG appears of particular interest as a prognostic pointer in DLBCL.27,31-35 However, no specific study has so far addressed PMBCL as a distinct subtype2-6 in which the metabolic response rate appears clearly inferior to that seen in other DLBCLs and Hodgkin lymphoma.7,8,36
Although the overall results of treatment of PMBCL are favorable,7,8 there remains a clear minority in whom the initial treatment fails, following which second-line treatment is frequently unsuccessful.9,10 In a retrospective series from the Princess Margaret Hospital in Toronto, the overall response rate to salvage chemotherapy (25% vs 48%) and 2-year OS after progression or relapse (15% vs 34%) were significantly inferior in 37 patients with relapsing or refractory PMLCL compared with a control group of 143 relapsing patients with other DLBCLs.9 In a retrospective Italian series, 138 consecutive patients were treated with the same chemotherapy combinations used in this study (CHOP or MACOP-B/VACOP-B) but without rituximab. Seventy-five percent of patients in complete remission received consolidation radiotherapy. Event-free survival and OS for patients treated with MACOP-B/VACOP-B were significantly better, but all the patients with stable disease or progression during chemotherapy (26) and all those relapsing after initial remission (12) died of lymphoma, irrespective of first-line and subsequent treatment type.10 In contrast to this finding, other studies have reported good outcomes in small groups of patients with chemosensitive relapse treated with high-dose therapy and autologous stem cell rescue.9,37 They showed that chemotherapy responsiveness immediately before transplantation is the strongest survival predictor. All these results highlight the need for studies focused on identifying poor-risk PMBCL patients to test novel induction and salvage strategies in advance of overt treatment failure.
Most patients with PMBCL present before the age of 60 years with early symptoms due to bulky mediastinal lesions that infiltrate the adjacent thoracic structures. For this reason, they rarely have other adverse prognostic factors.6 The IPI was originally developed for DLBCL, and studies of its utility in PMBCL have been contradictory.10,38-40 This may in part reflect differences in assigning stage when contiguous extranodal sites are involved.41 Moreover, the fact that most patients have elevated LDH reduces the usefulness of its discriminatory power and further affects the utility of IPI and aaIPI.38
As confirmed in the current series, conventional clinical prognostic indices for DLBCL, such as IPI and aaIPI, have only limited utility of identifying those who may benefit from different treatment strategies.8,12
We showed that TLG, which represents a combined assessment of tumor volume and metabolism on baseline 18FDG-PET/CT, was the most robust predictor of outcome not only as a dichotomized variable but also as a continuous parameter, irrespective of the cutoff point. The negative predictive power is extremely high, as there were no deaths and only 1 relapse among the patients with low TLG at diagnosis.
Our study was conducted in a large number of uniformly treated PMBCL patients. A small pilot study of 20 patients with DLBCL from the Massachusetts General Hospital also suggested that high baseline TLG may identify patients who are at increased risk of relapse after conventional R-CHOP34 . A survey from South Korea of 140 patients with DLBCL treated with R-CHOP showed that TLG is a better predictor of outcome than the IPI.27 Our study represents the largest prospectively collected series of PMBCL investigated with a central review of PET scans in which the quantitative functional parameters were retrospectively analyzed. The PET imaging was obtained following a strictly defined technical procedure, although detailed cross-calibration for equipment at the different centers was not possible. This may represent a limitation for a study focused on quantitative variables; however, our results are in keeping with the findings of the other studies from the United States and Korea and seem particularly promising for the early prediction of treatment failure in PMBCL.
The use of TLG in routine clinical practice would be premature, as there is a lack of a standardized methodology for the estimation of MTV and the TLG derived from this. Different methods and a wide range of threshold levels have been proposed to calculate the volume-based PET parameters either in solid tumors or in different subtypes of lymphoma (mainly DLBCL).25-35,42-45 Moreover, there are no published technical references about the methodology for the calculation of the volume-based PET parameters and definition of specific cutoff values in PMBCL.
Taking into account these points and the features of this disease, which is characterized by its thymic origin and presentation with a bulky FDG-avid mediastinal mass, we elected to experimentally define the PMBCL-specific volumetric PET parameters.
We estimated the MTV of PMBCL using an isocontour threshold method based on 25% of the SUVmax, which is lower than the 41% proposed by Boellard et al46 for the evaluation of tumoral lesions and tested by Meignan et al in Hodgkin lymphoma and DLBCL.44 However, our choice of a 25% threshold is justified by the very high FDG uptake currently found in the large and usually single PMBCL presenting mass (in our population, the median SUVmax was 18.8, with an interquartile range of 15.5 to 23) and is in keeping with other reports demonstrating that the ideal threshold (ie, the one that most accurately reflects the pathological tumor size) is inversely proportional to the lesion SUVmax.43,45 In this relationship, the best threshold is between 30% and 20% when SUVmax is between 20 and 30,45 and our results are in line with these findings.
We are aware that the use of a relatively low cutoff value may result in MTV overestimation in patients with small masses and reduced metabolic activity; however, this risk does not apply to this study population, where >85% of patients had a maximum tumor diameter >7 cm and only 4 patients had a SUVmax <11. Moreover, to mitigate potential bias, TLG was homogenously estimated in all patients during the central review of PET scans.
Hence, the effectiveness of TLG to risk stratify patients and the possibility of further improving the positive predictive value by combining TLG with other clinical and imaging parameters may warrant further studies. Indeed, this easily and early accessible parameter can help identify the few PMBCL patients who will eventually relapse and may be useful for designing trials aimed at improving their poor prognosis. In this setting, TLG may be a tool to select patients to be considered for elective front-line consolidation with myeloablative therapies. An alternative strategy for this group might be to use the dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin plus rituximab (DA-EPOCH-R) regimen, which has demonstrated very promising results7 but may not be necessary for the large proportion of patients who are probably curable with R-CHOP or R-MACOP-B/R-VACOP-B alone.8 Even if PET restaging at the end of chemotherapy can be used to reassess the risk of progression,8 a reliable baseline prognostic marker might allow us to determine who needs more complex infusional therapy and who is just as likely to be cured with the conventional regimens.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
Presented in part at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-20, 2013.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
A list of centers and investigators participating to the IELSG 26 study is reported in the online Appendix.
The authors thank Rita Gianascio Gianocca for her editorial assistance, the nursing and medical staff who looked after the patients at each center, and Dr Cassio P. De Campos for his critical reading of the manuscript and valuable review of the statistical analysis.
The study was endorsed by the Italian Lymphoma Foundation and by Cancer Research United Kingdom. We are grateful for the valuable contributions of Elena Porro, Monica Bellei, Marina Cesaretti, Kelly Cozens, and Carol J. Tyas to study coordination and data management. This study has been supported by grants ICP OCS-01709-04-2005 and ICP OCS-02062-03-2007 from Oncosuisse.
Authorship
L.C., E.Z., M.M., and P.W.M.J. designed the research and supervised the study; L.C., L.G., E.Z., M.M., P.W.M.J., and A.J.M.F. analyzed and interpreted data and wrote the manuscript; and all authors provided study materials or patients, contributed to the collection and assembly of data, discussed the results, critically reviewed the manuscript, and had final responsibility for the decision to submit.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emanuele Zucca, IELSG Operation Office, Ospedale San Giovanni, CH-6500 Bellinzona, Switzerland; e-mail: ielsg@ticino.com.
References
Author notes
L.C. and M.M. contributed equally to this study.
P.W.M.J. and E.Z. contributed equally to this study.