Key Points
SVT is a marker of occult cancer, in particular myeloproliferative neoplasms, liver cancer, and pancreatic cancer.
SVT is a prognostic factor for short-term survival in patients diagnosed with liver or pancreatic cancer.
Abstract
It is unknown if splanchnic venous thrombosis (SVT) is a marker of occult cancer and a prognostic factor for cancer survival. Using Danish medical registries, we conducted a nationwide cohort study including all patients with first-time SVT (n = 1191) between 1994 and 2011. We followed the patients for subsequent cancer diagnoses and calculated absolute risks and standardized incidence ratios (SIRs). We formed a matched comparison cohort of cancer patients without SVT, and assessed the prognostic impact of SVT on cancer survival by applying the Kaplan–Meier method and Cox regression. We followed the patients for a median of 1.6 years, and found that SVT was a marker of occult cancer. The 3-month cancer risk was 8.0% and the SIR was 33 (95% confidence interval, 27-40), compared with the general population. Increased risk was mainly found for liver cancer (risk = 3.5%; SIR = 1805), pancreatic cancer (risk = 1.5%; SIR = 256), and myeloproliferative neoplasms (risk = 0.7%; SIR = 764). The overall SIR remained increased twofold after 1 or more years of follow-up. SVT was also a prognostic factor for survival in patients with liver and pancreatic cancer. The clinical impact may be a more thorough diagnostic work-up in patients presenting with SVT.
Introduction
Venous thromboembolism may be a marker of occult cancer. Patients with a lower-limb deep venous thrombosis (DVT) or pulmonary embolism (PE) have a two- to fourfold increased risk of a cancer diagnosis in the first year after the thromboembolic event, compared with the general population.1-3 Recently, a similar association was demonstrated for superficial venous thrombosis.4 Patients, in whom thrombosis occurs before cancer diagnosis, are more likely to have advanced disease and higher mortality than cancer patients without venous thromboembolism at time of diagnosis.5 Splanchnic venous thrombosis (SVT) (ie, thrombosis of portal veins, hepatic veins [Budd-Chiari syndrome], mesenteric veins, and/or splenic veins)6 also may precede diagnosis of a malignant neoplasm. A few case reports have described SVT as the first sign of liver and pancreatic malignancies.7-9 A meta-analysis of 32 studies, each including between 10 and 237 patients with portal or hepatic vein thrombosis (HVT), showed that thrombosis often occurred prior to diagnosis of myeloproliferative neoplasms.10
The association between SVT and subsequent cancer risk has never been studied in a population-based setting using a comparison cohort. Moreover, the prognostic impact of SVT on cancer survival remains unknown.11 We therefore examined cancer risk after a first-time SVT diagnosis, compared with cancer risk in the general Danish population. In addition, we compared survival among cancer patients with and without SVT. The present study may extend our understanding of the development of SVT and may have implications for diagnostic work-up for cancer among patients presenting with this indication.
Methods
Data sources and study population
The Danish National Health Service provides tax-funded medical care to all Danish residents and guarantees free access to hospitals and outpatient clinics.12 We used data from the Danish National Patient Registry,13 recorded according to International Classification of Diseases (ICD) codes (8th and 10th revision). We identified all hospital inpatients and outpatients with a first-time ICD-10 code of SVT from 1994 through 2011. We retrieved information on comorbidities characterizing the patients from 1977 onwards, using ICD-8 and ICD-10 codes. We categorized the patients according to overall comorbidity level, using diseases included in the Charlson Comorbidity Index.14,15 We obtained information on diagnoses of liver disease (including varices and ascites), pancreatitis, diabetes, chronic obstructive pulmonary disease (as a proxy for smoking), venous thromboembolism (ie, DVT and PE), congestive heart failure, and myocardial infarction (MI) diagnosed at any time before SVT, and information on surgical procedures performed within 90 days before the thrombosis. We also retrieved registered abdominal ultrasound and computerized tomography (CT) scans performed within 30 days before or during the hospital contact with SVT. Registration of these diagnostic tests is complete since 2002.
Cancer outcomes
To identify patients with cancer, we linked the study cohort (using the patients’ unique personal identification number)16 to the Danish Cancer Registry,17 which contains data on prospectively recorded incident cancers diagnosed in Denmark since 1943, including month and year of diagnosis, and information on cancer stage at diagnosis. We searched for all cancer diagnoses, myeloproliferative neoplasms (including polycythemia vera, primary myelofibrosis, and essential thrombocytemia), and myelodysplastic syndromes (MDS).18 We excluded patients diagnosed with cancer (except for nonmelanoma skin cancer), myeloproliferative neoplasm, or MDS before the diagnosis date of SVT.
In the prognostic analysis, we examined survival among patients in our cohort who were later diagnosed with liver cancer, pancreatic cancer, or myeloproliferative neoplasm, and compared this with survival among matched cancer patients without SVT. We used the Danish Cancer Registry to identify up to five comparisons for each patient, matched by cancer type and stage (except for myeloproliferative neoplasm as there is no standard staging system), sex, age (5-year intervals), and year of diagnosis (5-year intervals).
All diagnosis codes and variable categorizations used are provided in the supplemental Appendix, available on the Blood Web site.
Statistical analysis
Descriptive data are presented as frequencies or as median values with interquartile ranges (IQRs). We followed each patient from date of first diagnosis of SVT until date of cancer diagnosis, emigration, death, or December 31, 2011, whichever came first.
We computed the absolute risk (cumulative incidence) of cancer in patients with a SVT diagnosis, treating death as a competing risk. Standardized incidence ratios (SIRs) (with 95% confidence intervals [CIs]) were used as a measure of relative risk, comparing cancer incidence observed among patients with SVT with that expected based on national cancer incidence rates by age, sex, and calendar year. SIRs were stratified by: patient characteristics, type of thrombosis, primary and secondary diagnoses, covariates, and cancer stage. We repeated the analyses for the subgroup of patients who had an ultrasound or CT scan within 30 days before or during their hospital contact with SVT.
The survival analysis was restricted to the most frequent cancers in the study cohort. We characterized the patients according to diseases occurring before their cancer diagnosis.
We summarized survival of cancer patients, by constructing Kaplan–Meier survival curves. We used Cox proportional hazard regression to compare risk of death among cancer patients with and without SVT, by computing mortality rate ratios and associated 95% CIs (adjusting for cancer type and stage, sex, age, and year of diagnosis).
All statistical analyses were conducted using the SAS statistical software package, version 9.2 (SAS Institute, Cary, NC). The study was approved by the Danish Data Protection Agency, record #1-16-02-1-08. Danish registry data are generally available to researchers. According to Danish law, the use of registry data for research purposes does not require informed consent.
Results
Risk analysis
Patient characteristics.
We identified 1191 patients with SVT; 924 (78%) had portal vein thrombosis (PVT), 141 (12%) had HVT, and 126 (10%) had mesenteric thrombosis. Median age was 61 years (46-74 years) and 52% were men. Nearly all patients, 1026 (86%) received their thrombosis diagnosis during a hospital admission, whereas only 165 (14%) were diagnosed in an outpatient clinic.
The majority of patients in our cohort had a moderate (34%) or severe (23%) level of comorbidity. In particular, we found a high prevalence of liver disease (20%), diabetes (15%), heart disease (15%), and previous pancreatitis (12%). In addition, 33% of the patients had undergone a surgical procedure less than 90 days prior to their thrombotic event (Table 1). Information on cancer stage was available for 111 (74%) of the 150 patients with nonhematologic cancers. Of these, 52 (47%) had localized cancer and 59 (53%) had regional spread or distant metastasis.
Overall cancer risk.
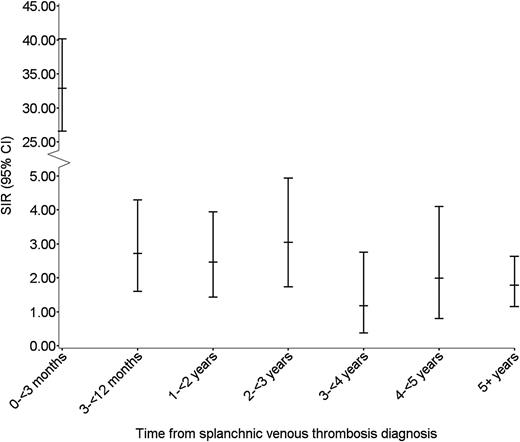
During median follow-up of 1.6 years (IQR, 0-5 years), we identified 183 incident cancers, corresponding to an overall SIR of cancer of 4.2 (95% CI, 3.6-4.9). The majority of cancers were diagnosed among patients with PVT (n = 161, 88%), with an overall SIR of 4.7 (95% CI, 4.0-5.5) (Table 2). In total, 21 cancers (11%) were diagnosed among patients with HVT, corresponding to an overall SIR of 2.9 (95% CI, 1.8-4.4) (Table 2). One cancer was diagnosed in a patient with mesenteric vein thrombosis. During the first 3 months of follow-up, 95 cancers were diagnosed and among these, 53 were diagnosed within the first month. Three-month and 5-year absolute risks of cancer among SVT patients were 8.0% and 14.8%, respectively. During the first 3 months of follow-up, the SIR was 33 (95% CI, 27-40); between 3 and 12 months the ratio was 2.7 (95% CI, 1.6–4.3); and beyond 1 year of follow-up it remained increased twofold, compared with the risk in the general population (Table 1; Figure 1).
We observed no difference in cancer risk between men and women. Although the majority of cancers were diagnosed in patients older than 40 years, the excess risk was more pronounced in patients younger than age 40. The risk of cancer subsequent to SVT increased during the study period, which likely reflected improved diagnostics with a higher accuracy of diagnoses. Between 1994 and 1999, the SIR was 3.0 (95% CI, 2.2-4.1) and between 2006 and 2011 it was 6.0 (95% CI, 4.8-7.5).
SVT was the primary reason for the hospital contact for 674 patients (57%). Stratification by thrombosis as the primary vs secondary reason for admission yielded SIRs of 3.8 (95% CI, 3.1-4.6) and 4.9 (95% CI, 3.9-6.1), respectively. Patients with liver disease, diabetes, or recent surgery were at higher risk of cancer than patients without these diseases or recent surgery (Table 1). In sub-analyses based on patient characteristics, only chronic obstructive pulmonary disease modified the SIRs after more than 1 year of follow-up (data not presented).
Liver and pancreatic cancer.
The increased cancer risk during the first 3 months following an SVT diagnosis stemmed mainly from excess risk of liver cancer (absolute risk = 3.5%; SIR = 1805 [95% CI, 1295-2448]) and pancreatic cancer (absolute risk = 1.5%; SIR = 256 [95% CI, 149-409]), and occurred in patients with PVT. Although the prevalence of liver disease in the overall cohort was 20%, it was present in 50% of the patients diagnosed with liver cancer. Only 4 (20%) of the 20 patients with pancreatic cancer had previous pancreatitis. Of note, among patients diagnosed with liver cancer with known stage during the first 3 months following the thrombotic event, 16 had localized cancer (SIR = 2451 [95% CI, 1400-3981]) and 9 had advanced cancer (SIR = 1191 [95% CI, 546-2263]). Among patients diagnosed with pancreatic cancer, 2 had localized cancer (SIR = 227 [95% CI, 27-820]) and 11 had advanced cancer (SIR = 263 [95% CI, 131-470]). We found a persistent increased cancer risk beyond 3 months of follow-up, but the estimates were imprecise (Table 3).
Hematologic cancer.
The majority of hematologic cancers diagnosed in our cohort was myeloproliferative neoplasms, and were diagnosed among patients with HVT. The absolute risk of a myeloproliferative neoplasm diagnosis during the first 3 months was 0.7% and the SIR was 764 (95% CI, 329-1505) (Table 3). Beyond 1 year of follow-up, the patients still had a pronounced excess risk of myeloproliferative neoplasms (SIR = 88 [95% CI, 45-153]). After 5 years of follow-up, the absolute risk of myeloproliferative neoplasms was 2.2%, and at end of follow-up it was 3.5%. We also observed an excess risk of lymphoma, leukemia, and MDS during the first 3 months of follow-up. Thereafter, the risk did not differ from the expected risk (Table 3).
Other cancers.
The number of lung, stomach, gallbladder/biliary tract, and urinary tract cancers observed during follow-up in patients diagnosed with SVT was higher than expected. The overall risk of being diagnosed with these smoking-related cancers was increased threefold to 14-fold compared with the expected (Table 3). Cancers of the colon, rectum, breast, uterus, and prostate were only weakly or not associated with SVT (Table 3).
Patients with ultrasound and/or CT scan-confirmed diagnosis of SVT.
Among the 881 patients diagnosed with SVT after 2002, 624 events (71%) were confirmed by abdominal ultrasound and/or CT scan. In this subgroup, the overall cancer risk was even higher (7.7 [95% CI, 6.3-9.4]) than for the entire SVT cohort (Table 1). During the first 3 months of follow-up, the SIR for cancer was 52 (95% CI, 41-66); between 3 and 12 months of follow-up, the ratio was 4.3 (95% CI, 2.2-7.5); and beyond 1 year of follow-up it remained increased twofold. The proportion of SVT confirmed by ultrasound or CT scan increased from 66% in 2002 to 85% in 2011. For patients with a confirmed diagnosis between 2002 and 2006, the overall cancer SIR was 4.7 (95% CI, 3.4-6.5), and between 2007 and 2011 it was 12 (95% CI, 9.4-15).
Survival analysis
Characteristics.
The survival analyses included 259 patients with liver cancer, 116 patients with pancreatic cancer, and 107 patients with myeloproliferative neoplasms. Among these patients, SVT preceded the cancer diagnosis in 48 (all with PVT), 20 (19 with PVT and 1 with HVT), and 23 (15 with PVT and 8 with HVT) patients, respectively. Compared with matched cancer patients without SVT, more patients diagnosed with SVT before their cancer diagnosis had a high comorbidity level, including liver disease and associated complications, diabetes, and more had undergone surgical procedures within 90 days (Table 4).
Survival.
Patients with liver or pancreatic cancer had a poor outcome, regardless of presence of SVT before cancer diagnosis (Figure 2A-B).
Survival curves for cancer patients with and without SVT. (A-C) Survival curves for patients with a diagnosis of liver cancer (A), pancreatic cancer (B), or myeloproliferative neoplasm (C) and SVT, and for a matched comparison cohort of cancer patients without SVT (matched by cancer type and stage, sex, age [5-year intervals], and year of diagnosis [5-year intervals]).
Survival curves for cancer patients with and without SVT. (A-C) Survival curves for patients with a diagnosis of liver cancer (A), pancreatic cancer (B), or myeloproliferative neoplasm (C) and SVT, and for a matched comparison cohort of cancer patients without SVT (matched by cancer type and stage, sex, age [5-year intervals], and year of diagnosis [5-year intervals]).
The 3-month survival after liver cancer diagnosis was 44% for patients with and 55% for patients without SVT, corresponding to a mortality rate ratio of 1.5 (95% CI, 0.9-2.3). After 1 year of follow-up, thrombosis was still a prognostic factor for liver cancer patients; survival was 17% among patients with thrombosis and 30% among patients without thrombosis. At the end of follow-up, the mortality rate ratio for liver cancer was 1.6 (95% CI, 1.1-2.3).
SVT was also a prognostic factor for patients with pancreatic cancer. The 3-month survival after pancreatic cancer diagnosis was 35% for patients with and 53% for patients without SVT, yielding a 3-month mortality rate ratio for pancreatic cancer of 1.5 (95% CI, 0.8-2.9). Among patients with pancreatic cancer, SVT was not a prognostic factor for 1-year survival (15% for patients with and 17% for patients without thrombosis). The overall mortality rate ratio for pancreatic cancer was 1.4 (95% CI, 0.8-2.5).
In contrast, patients with myeloproliferative neoplasms had a much better prognosis (Figure 2C), regardless of the presence of an SVT. Due to the few deaths among these patients, we did not analyze the impact of SVT on relative mortality.
Discussion
In this cohort study, we found SVT to be a strong marker of occult cancer. In particular, we observed a higher incidence of liver cancer, pancreatic cancer, and myeloproliferative neoplasms than expected during the first 3 months after a PVT or HVT diagnosis. Although excess cancer occurrence decreased after 3 months, SVT remained a marker of slightly increased cancer risk during subsequent follow-up, especially for myeloproliferative neoplasms. SVT was a prognostic factor for short-term survival in patients with liver and pancreatic cancer, but did not impact survival in patients with myeloproliferative neoplasms.
The pathogenesis of cancer-related SVT includes cancer-associated hypercoagulability, vessel-wall injury (tumor invasion), and stasis (splanchnic vein compression).19 Our finding of a greatly increased short-term risk of cancer in patients with SVT may have several explanations. The substantial fall in risk after 3 months of follow-up implies that cancer preceded the thrombosis. An unrecognized malignancy likely triggered thrombus formation, and in some patients it may have been the first sign of cancer. Supporting this assumption, we found that more patients had SVT registered as the primary, rather than secondary, reason for their hospital contact. In other patients, the thrombosis may have been coincidentally detected in the diagnostic work-up for cancer,11 which could be the case for patients diagnosed with both diseases during the first month of follow-up. The persistent increased risk of liver cancer is likely related to underlying diseases such as liver cirrhosis,20 whereas the increased risk of myeloproliferative neoplasms beyond 1 year of follow-up may indicate that diagnosis of these neoplasms was delayed.21 We had no information on test results for the JAK2V617F mutation, but it is possible that the finding of this mutation was related to diagnosis of myeloproliferative neoplasms in some patients.10 Alcohol abuse is a risk factor for SVT, but is also associated with smoking.22 Because smoking is a strong risk factor for cancer,23 a combination of alcohol abuse and smoking may be the link behind the increased risk observed for lung, stomach, and bladder cancers. The increased risk of cancer during the study period likely reflects improved diagnostics, with more frequent use of CT scans.
Our study was conducted in a setting in which a national health service provides unfettered access to health care, allowing us largely to avoid referral and selection biases.24 Other strengths were our inclusion of the entire Danish population and complete individual-level follow-up through access to patients’ full hospital histories, as well as to outpatient clinic histories since 1994. Whereas diagnoses in the Danish Cancer Registry generally have high validity, with up to 95% to 98% completeness and accuracy of recorded diagnoses,13,17 the registration of SVT in the Danish National Patient Registry has not been validated previously. We sought to strengthen the validity of SVT diagnoses by including only those registered with a specific anatomic location (excluding unspecified abdominal venous thrombosis). Moreover, we found that the majority (71%) of SVT diagnoses in our cohort were based on ultrasound examinations or CT scans, and hence were confirmed diagnoses. Finally, the use of registry data precluded detailed information on clinical care of patients.
Screening with abdomino-pelvic ultrasound, CT, or fluoro-2-deoxy-d-glucose–positron emission tomography combined with CT increases the chance of detecting an occult cancer in patients with venous thromboembolism.25,26 The most recent guideline by the United Kingdom National Institute for Health and Clinical Excellence (NICE CG144; 2012), recommends considering an abdomino-pelvic CT scan in patients aged over 40 years presenting with venous thromboembolism.27 We speculate if abdominal CT or PET/CT scans should be mandatory in the diagnostic work-up in patients with SVT. Nevertheless, proposals for implementing new diagnostic work-up procedures for occult cancer are only reasonable if they improve cancer-associated survival and are cost-effective. Based on the existing literature, screening for occult cancers in patients with lower-limb DVT and PE may help identify cancers at an early stage, but does not necessarily improve cancer-related survival.28 However, the detection of underlying cancer potentially influences the management of venous thromboembolism,29 as recurrence and complications are more frequent among cancer patients.30,31
In conclusion, we found evidence that SVT is a strong marker of occult cancer and a predictor of poor prognosis for patients with liver and pancreatic cancer.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.K.S. and H.T.S. conceived the study idea, designed the study, and directed the analyses, which were carried out by D.K.F. and L.P.; K.K.S. reviewed the literature, organized the writing, and wrote the initial drafts; and all authors participated in the interpretation of the results, critically revised the manuscript for intellectual content, and approved the final version.
The study was supported by the Clinical Epidemiology Research Foundation, Denmark; Aarhus University Research Foundation; the Karen Elise Jensen Foundation; and by a grant from the Danish Cancer Society (R73-A4284-13-S17). The study sponsors had no influence on the study design, collection, analysis, and interpretation of the data or in the writing of the report. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirstine K. Søgaard, Department of Clinical Epidemiology, Aarhus University Hospital, Olof Palmes Allé 43-45, 8200 Aarhus N, Denmark; e-mail: kks@clin.au.dk.

![Figure 2. Survival curves for cancer patients with and without SVT. (A-C) Survival curves for patients with a diagnosis of liver cancer (A), pancreatic cancer (B), or myeloproliferative neoplasm (C) and SVT, and for a matched comparison cohort of cancer patients without SVT (matched by cancer type and stage, sex, age [5-year intervals], and year of diagnosis [5-year intervals]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/8/10.1182_blood-2015-03-631119/3/m_957f2.jpeg?Expires=1766129192&Signature=ZXdShS7-1WSm1vMBHov3KnV06KHI6yJuKmP8omDdttsfMqQ9Oxiasey5XWJ127z5aBF9o578pKM0Rhe9J782VMefSTamzfn547RP6mtiXdkLGbHV9ZrhC2dzie4V~IVaGwaSZot-B5qWHAoSkkT-35oZjUgV1uy9JH-axyfoenGjsxjJu2SLb9ikeUBOsbdPRclWAa7FFtX9RYPD9~-JejqY3D5mJaiJXd2OZtK-7z8ktdi37~lB2gcPUtAnrbiI52u7Q0WEDr9mOJy68QJUnYWEXDJbxF3qf21RPuqvtdE6JclIPBQuUwHgn73X~ioZ8LpzhfjPcbmXT1dKVm4T~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
