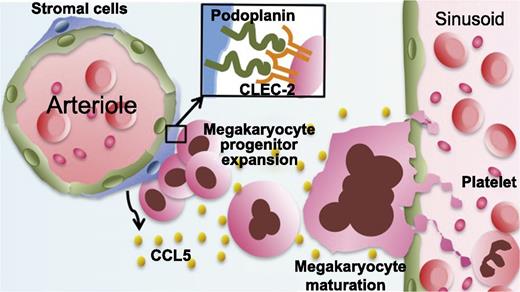
In this issue of Blood, Tamura et al reveal a novel function for podoplanin on periarteriolar stromal cells in the bone marrow: promoting megakaryocyte growth and proplatelet formation by interaction with C-type lectin-like receptor 2 (CLEC-2).1
Model showing that megakaryocyte CLEC-2 partners with podoplanin on periarteriolar stromal cells to promote megakaryopoiesis and thrombopoiesis.
Model showing that megakaryocyte CLEC-2 partners with podoplanin on periarteriolar stromal cells to promote megakaryopoiesis and thrombopoiesis.
CLEC-2 is a type II transmembrane protein that was initially identified through a bioinformatic search as being homologous to the C-type lectin-like receptors on natural killer cells.2 CLEC-2 is primarily expressed on dendritic cells and platelets. On dendritic cells, CLEC-2 functions dually: it facilitates the migration of activated dendritic cells to, and also attenuates the contractility of fibroblastic reticular cells in, the draining lymph nodes.3 On platelets, CLEC-2 was first identified as a receptor for a snake venom toxin, rhodocytin, that elicits aggregation of platelets through activation of Src and Syk nonreceptor tyrosine kinases.2 However, the physiologic function and physiologically relevant ligand of platelet CLEC-2 remained elusive until it was found to be the receptor for the O-glycosylated protein podoplanin,2 which is also known as Aggrus, T1α, and gp38, depending on the cell type in which the molecule was first found.
Podoplanin is a type I transmembrane O-glycoprotein. Under normal conditions, it is expressed on many cell types, including lymphatic endothelial cells and fibroblastic reticular cells in the lymph node. The first physiologic function of podoplanin and CLEC-2 interaction was serendipitously uncovered by characterizing mice lacking glycosyltransferase T-synthase (encoded by C1galt1), which controls the mucin-type O-glycosylation.4 Mice lacking endothelial T-synthase and podoplanin shared the same defective blood and lymphatic vessel separation during embryonic development.4 Further studies showed that the blood/lymphatic vessel–mixing phenotype was also present in mice lacking platelet CLEC-2,5 indicating that activation of platelet CLEC-2 by binding O-glycosylated podoplanin is essential for blood/lymphatic separation. During early development, podoplanin is also highly expressed by neural progenitor cells. Mouse embryos lacking either podoplanin or platelet CLEC-2 exhibit brain hemorrhages, indicating that pairing of these 2 molecules protects neurovascular integrity in the developing brain as well.6 After birth, platelet CLEC-2 was found to maintain the separated blood-lymphatic system by interacting with podoplanin at the lymphovenous junction.7 Furthermore, platelet CLEC-2 was discovered to be critical for the integrity of high endothelial venules by interacting with podoplanin on fibroblastic reticular cells in the lymph node during lymphocyte trafficking.8
CLEC-2 is also highly expressed on megakaryocytes, the parent cells of platelets. In the bone marrow, CLEC-2 signaling in megakaryocytes was recently found to be important for the maintenance of hematopoietic stem cells by mediating the production of thrombopoietin from megakaryocytes in the bone marrow stem cell niche.9 However, the activating ligand for megakaryocyte CLEC-2 and its cellular source remain unclear. Tamura and colleagues discovered that podoplanin is expressed on stromal cells surrounding arterioles in the bone marrow, that immature megakaryocytic colonies form adjacent to the podoplanin-positive stromal cells, and that podoplanin promotes megakaryocyte growth and proplatelet formation in vitro by interacting with CLEC-2. In addition, they revealed that the binding of megakaryocyte CLEC-2 to podoplanin results in chemokine (C-C motif) ligand 5 (CCL5) secretion from bone marrow stromal cells, which is associated with increased proplatelet formation (see figure).
Megakaryopoiesis and thrombopoiesis are complex and hierarchical processes. Tamura et al’s newly discovered function of CLEC-2 on megakaryocytes interacting with podoplanin on fibroblastic reticular cell–like stromal cells provides an important insight into a regulatory process for platelet production in the bone marrow. Although there are likely differences between humans and mice with regard to the fine details of the system, which require further studies, this new understanding of megakaryopoiesis and platelet production may bring us a step closer to manufacturing platelets in vitro for clinical applications.
Conflict-of-interest disclosure: The authors declare no competing financial interests.