Key Points
Specific interaction of DF with EC membranes is followed by its internalization mainly through macropinocytic mechanisms.
DF attachment to the cell membrane is sufficient to perform its antiinflammatory and antioxidant effects on the endothelium.
Abstract
Defibrotide (DF) has received European Medicines Agency authorization to treat sinusoidal obstruction syndrome, an early complication after hematopoietic cell transplantation. DF has a recognized role as an endothelial protective agent, although its precise mechanism of action remains to be elucidated. The aim of the present study was to investigate the interaction of DF with endothelial cells (ECs). A human hepatic EC line was exposed to different DF concentrations, previously labeled. Using inhibitory assays and flow cytometry techniques along with confocal microscopy, we explored: DF-EC interaction, endocytic pathways, and internalization kinetics. Moreover, we evaluated the potential role of adenosine receptors in DF-EC interaction and if DF effects on endothelium were dependent of its internalization. Confocal microscopy showed interaction of DF with EC membranes followed by internalization, though DF did not reach the cell nucleus even after 24 hours. Flow cytometry revealed concentration, temperature, and time dependent uptake of DF in 2 EC models but not in other cell types. Moreover, inhibitory assays indicated that entrance of DF into ECs occurs primarily through macropinocytosis. Our experimental approach did not show any evidence of the involvement of adenosine receptors in DF-EC interaction. The antiinflammatory and antioxidant properties of DF seem to be caused by the interaction of the drug with the cell membrane. Our findings contribute to a better understanding of the precise mechanisms of action of DF as a therapeutic and potential preventive agent on the endothelial damage underlying different pathologic situations.
Introduction
Defibrotide (DF) is a mixture of 90% single-stranded phosphodiester oligonucleotides (length, 9-80 mer; average molecular mass, 16.5 ± 2.5 KDa) and 10% double-stranded phosphodiester oligonucleotides, derived from the controlled depolymerization of porcine intestinal mucosal DNA.1-3 Several functions, specially related to hemostasis, have been ascribed to DF.4 In this regard, our group has demonstrated the protective effect of DF on the endothelium, by preventing the endothelial damage associated with hematopoietic cell transplantation (HCT) conditions,5,6 and with the deleterious effect of immunosuppresants.7 In our in vitro endothelial activation model, DF has exhibited reproducible effects on endothelial cells (ECs) from different origins. DF demonstrates antiinflammatory, antithrombotic, and antiapoptotic properties. However, although its effects are increasingly better understood, its precise mechanism of action remains to be elucidated.
There is limited knowledge about DF pharmacokinetics, pharmacodynamics, and mechanisms of action.8-10 However, 2 distinct properties of DF (endothelial protection and restoration of the thrombotic-fibrinolytic balance) were key to test its effect on the sinusoidal obstruction syndrome (SOS), a life-threatening complication associated with HCT.11 Results from several studies carried out over the last 15 years, 2 trials aimed to evaluate the effect of DF on SOS,12,13 and our in vitro studies5,7,14 led to its approval for the treatment of severe SOS and the orphan designation for the prevention of graft-versus-host disease (GVHD) in European countries by the European Medicines Agency in 2013.
HCT is a well-established approach for the treatment of several hematologic malignancies and other nonmalignant disorders.15 Although it has a beneficial effect, HCT is associated with several early and late life-threatening complications. EC activation seems to be a common pathogenic mechanism in several early HCT complications. The endothelium is an active biological interface between the blood and all other tissues, with a variety of functions throughout the circulatory system. Several input stimuli may produce local or systemic physiologic endothelial activation. EC activation includes a wide spectrum of phenotypic changes in the different locations of the vascular bed. When the activating stimulus is too intense or persistent, it may result in a dysfunctional endothelium, potentially leading to a net liability to the host with single- or multiorgan failure.16,17
At the time of DF discovery, the concept of “one drug–one activity” was still dominant in the field of pharmacology. Currently, this idea is progressively evolving to the notion of “multitarget compound,” which fits perfectly with DF. The huge variety of the properties ascribed to DF could also be grouped in a more global concept such as EC protective drug. Considering that dysfunction of the liver endothelium is the key trigger element for SOS development in HCT, our hypothesis is that DF interacts specifically with ECs, enhancing its resistance to several injuries. The aim of the present study was to define the mechanisms of action of DF on the endothelium by using an EC line with hepatic origin. Experiments were designed to ascertain at which cellular level DF is undertaking its protective action on ECs.
Methods
Experimental design
The interaction of DF with hepatic EC compartments was evaluated to determine whether the interaction is restricted to the cell membrane or if it is internalized into the cytoplasm. ECs were incubated with DF (4 μg/mL) previously labeled with a nucleic acid dye, following manufacturer instructions (UlysisAlexa Fluor 488 Nucleic Acid Labeling Kit, Life Technologies, Carlsbad, California). The kinetics of DF–ECs interaction and inhibitory assays were assessed by flow cytometry. The specific location of DF was identified by confocal microscopy. The role of adenosine receptors for DF binding was explored by using adenosine receptor antagonist (8-p-sulfophenyltheophylline).
The physiologic relevance of DF redistribution along the EC membrane or the cytoplasm was explored by measuring the potential protective effect of DF on the endothelium regarding the inflammation caused by cyclosporine A (CSA), the production of reactive oxygen species (ROS) by H2O2, and the cell death induced by oxidative stress.
To investigate the specificity of DF–ECs interaction, peripheral blood mononuclear cells (PBMCs) and a primary human umbilical vein endothelial cell line (HUVEC) were also used.
Cell culture and reagents
SK-HEP1 (SK) (ATCC, Manassas, VA), an immortal human cell line with endothelial origin derived from the ascitic fluid of a patient with liver adenocarcinoma,18,19 was grown at 37°C in a 5% CO2 humidified incubator in Eagle’s Minimum Essential Medium (ATCC) supplemented with 100 U/mL penicillin, 100 g/mL streptomycin, and 10% fetal bovine serum (Gibco BRL, Life Technologies, Scotland, UK). Culture media was replaced every 2 days. Cells were used between the tenth and fifteenth passages.
HUVEC were isolated from human umbilical veins as previously described,20 and were maintained and subcultured (37°C, 5% CO2) in Medium 199 (Gibco BRL) supplemented with 100 U/mL penicillin, 100 g/mL streptomycin, and 20% pooled human sera obtained from healthy donors. Culture media was replaced every 2 days. Cells were used between the second and third passages.
PBMCs were derived from heparinized (BD Vacutainer, UK) blood of healthy human volunteers according to a standard protocol21 using Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density-gradient centrifugation.
DF uptake and inhibitory assays through flow cytometry
Uptake of fluorophore-labeled DF by ECs was determined by incubating cells with DF for 4 hours. Then cells were extensively washed, trypsinized, and analyzed in the flow cytometer (Navios, Beckman Coulter, Inc.). For saturable assays, SK were exposed to increasing doses of DF (0 to 12μg/mL, 2 hours). For temperature dependence assays, SK were incubated with 4μg/mL, 2 hours, at 37°C and 4°C.22 To explore the role of adenosine receptors, 10μM 8-p-sulfophenyltheophylline (8PS) (Sigma-Aldrich) was added to the culture 1 hour before incubation with DF.3 Only alive cells, Topro3 negative (Life Technologies), were considered for the analysis.
Several inhibitory strategies were performed in the flow cytometry experimental model.23 Before adhesion of DF to the culture, SK were exposed for 1 hour to endocytosis and cell traffic inhibitors (all from Sigma-Aldrich) at the maximum concentration that led to <10% of cell death24 : 25 μM chlorpromazine and 100 µM indomethacin for clathrin and caveoline-mediated endocytosis; 100 nM Wortmannin and 250 μM amiloride for macropinocytosis; and 20 μM cytochalasin D and 25 μM nocodazole as trafficking inhibitors of actin- and microtubules-dependent movement.
Fixed-cell confocal fluorescence imaging
ECs were grown in Ibidi 15 μ-Slide 8-wells plaques and incubated with fluorophore-labeled DF (4 μg/mL) at 37°C along different time points. Then cells were washed, fixed with 4% of paraformaldehyde, and used in immunofluorescence assays. Wheat germ agglutinin (Life Technologies) and Hoechst (Life Technologies) were used for membranes and nuclei staining, respectively. Cells were permeabilized with 0.1% saponin (10 minutes), blocked with 0.2% bovine serum albumin, and incubated with a rabbit anti-caveoline antibody (Becton Dickinson, Madrid, Spain) and a mouse anti-clathrin antibody (ABR Affinity BioReagents, Golden, CO), both visualized with a secondary IgG conjugated with Alexa 647 (Thermo Fisher, Waltham, MA), and LysoTracker for lysosomes staining (Life Technologies). Confocal images were captured using a Leica TCS SP5 microscope and a 63× immersion objective. Optical sections (z) were performed each 2 μm. Image analysis was carried out using Fiji software (National Institutes of Health, Bethesda, MA).
Immunofluorescence detection of VCAM-1 expression in cell monolayers
Cells were seeded into 6-well plates containing 18 × 18 mm2 coverslips and exposed to CSA (final concentration of 200 ng/mL), an immunosuppressive drug that induces endothelial damage,7 for 24 hours. Cells were coincubated with DF and Wortmannin for 24 hours before exposure to the immunosuppressant (Wortmannin was added 1 hour before DF). VCAM-1 expression was evaluated as previously described.25 Fluorescence micrographs were analyzed using ImageJ version 1.43m (National Institutes of Health, http://rsb.info.nih.gov/nih-image/manual/tech.html#). Cultured cells were selected from the background with the threshold tool, and the fluorescence intensity was measured only in the selected area. Results are expressed as average fold increases of mean gray value of each condition vs the control.
Production of intracellular ROS and oxidative stress–induced cell death
ROS generation was explored using the cell-permeable ROS detection reagent 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2 DCFDA) (Molecular Probes).25 ECs seeded on 24-well plates (TPP, Sigma-Aldrich) were incubated with 2μ M CM-H2DCFDA and 1 μM H2O2 (Sigma-Aldrich) at 37°C for 45 minutes in dark conditions in the presence of DF (500 μg/mL) and with or without the macropinocytosis inhibitor Wortmannin (100 nM). Ten minutes before analysis of SK by flow cytometry, 1 μM Topro3 was added. Intracellular ROS production was evaluated by flow cytometry only in living cells.
A similar experimental approach was applied, exposing cells to a high concentration of H2O2 (50 μM) to evaluate the protective effect of DF to cell death induced by oxidative stress. Wortmannin was used to determine in which cell compartment DF was acting.
Activation of inflammation and oxidative cell signaling pathways in ECs
SK cells grown in 6-well plates incubated or not with DF and/or Wortmannin for 24 hours were exposed to CSA (5 minutes) to evaluate changes in the phosphorylation of p38MAPK,6 or to 1 μM H2O2 (24 hours) to investigate alterations in endothelial nitric oxide synthase 3 (eNOS3) synthesis. SK were lysed with Laemmli’s buffer, sonicated to shear DNA and reduce viscosity (15 s), and heated to 90°C (5 minutes). Protein concentration in the supernatants was determined using Coomassie Plus (Pierce, Barcelona, Spain). Samples were resolved by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, proteins transferred to nitrocellulose membranes, and probed with specific antibodies against p38MAPK, eNOS3, and β-actin (Cell Signaling, Danvers, MA). Membranes were incubated with a peroxidase-conjugated anti-rabbit IgG and developed by chemiluminescence.
Statistical analysis
Results are expressed as mean ± standard error of the mean. Statistical analysis was performed with raw data using Student t test for paired samples and analysis of variance. Results were considered statistically significant when P < .05. SPSS statistical package 17.0.0 (SPSS Inc./IBM, Armonk, NY) was used for all analyses.
Results
Time, concentration, and temperature dependency of DF uptake by ECs
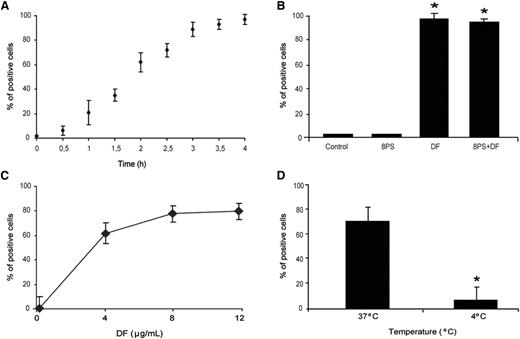
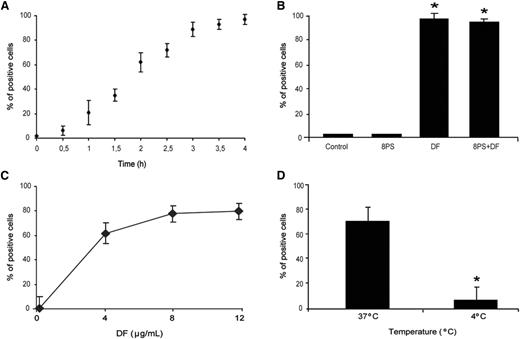
DF interaction with ECs was evaluated by flow cytometry. DF–SK interaction was time dependent with a linear increase of DF uptake by SK for up to 4 hours, after which 97% ± 4% of cells were positive for DF-associated fluorescence, with a mean fluorescence intensity of 7 ± 0.5 (Figure 1A). Blockade of adenosine receptors with 8PS did not prevent DF–SK interaction (94% ± 3% of cells were positive for DF-associated fluorescence; n = 4 and P < .05 compared with both control cells and SK exposed to 8PS alone) (Figure 1B). Dose-response experiments showed saturable DF interaction, with a maximum at 8 μg/mL (83% ± 10% of cells were positive after 2 hours of incubation), although no significant statistical difference was found when compared with cells exposed to 4 μg/mL (65% ± 8%) (Figure 1C). Experiments performed with SK incubated with DF at 4°C for 2 hours demonstrated that DF–SK interactions are temperature-dependent (positive cells of 9% ± 4% at 4°C compared with 65% ± 8% at 37°C; P < .05, n = 6) (Figure 1D).
Uptake of defibrotide by endothelial cells. (A) SK cells were incubated with DF (4 μg/mL final concentration), labeled with Alexa 488, for the indicated period of time (from 0-4 h). (B) The addition of 10 μM adenosine receptor antagonist (8-p-sulfophenyltheophylline) to SK monolayers for 1 hour before the incubation with DF (4 h) did not interfere with SK–DF interaction. (C) For saturation assays, SK monolayers were exposed to increasing doses of DF (from 0-12 μg/mL) for 2 hours. (D) For temperature dependence assays, SK were incubated with 4 μg/mL for 2 hours at 37°C and 4°C. Results are expressed in percentage of positive cells.
Uptake of defibrotide by endothelial cells. (A) SK cells were incubated with DF (4 μg/mL final concentration), labeled with Alexa 488, for the indicated period of time (from 0-4 h). (B) The addition of 10 μM adenosine receptor antagonist (8-p-sulfophenyltheophylline) to SK monolayers for 1 hour before the incubation with DF (4 h) did not interfere with SK–DF interaction. (C) For saturation assays, SK monolayers were exposed to increasing doses of DF (from 0-12 μg/mL) for 2 hours. (D) For temperature dependence assays, SK were incubated with 4 μg/mL for 2 hours at 37°C and 4°C. Results are expressed in percentage of positive cells.
Evidence of DF internalization and specific uptake by ECs
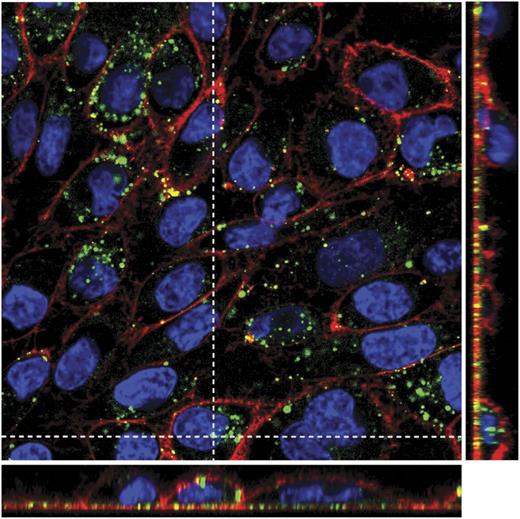
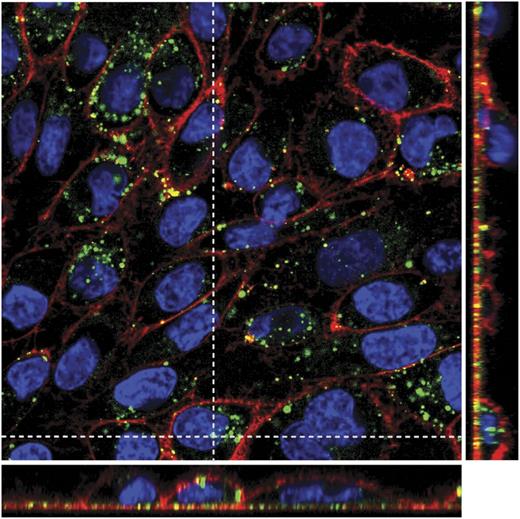
SK monolayers exposed to DF (for 24 hours) showed a vesicular staining pattern of internalized DF in the cell cytoplasm (Figure 2). Merged images of all individual channels (red staining for membranes, blue for nuclei, and green for DF) and Z projection (narrow images in the right and in the bottom of the main picture) show that DF remains in the cytoplasm and does not enter into the nuclei, at least during 24 hours of incubation. (Supplemental Video, available on the Blood Web site, shows the first 45 minutes of DF-EC interaction.)
DF internalization by SK cells. Merged confocal images of all individual channels (red staining with wheat germ agglutinin for membranes, blue with Hoechst for nuclei, and green with Alexa 488 for DF), and Z projection (narrow images in the right and in the bottom of the main picture) show that DF remains in the cytoplasm displaying vesicular staining and does not enter into the nuclei, at least after 24 hours of incubation. Confocal images were taken using a Leica TCS SP5 microscope and a 63× oil immersion objective. Optical sections (z) were performed each 2 μm. Image analysis was performed using Fiji software (National Institutes of Health).
DF internalization by SK cells. Merged confocal images of all individual channels (red staining with wheat germ agglutinin for membranes, blue with Hoechst for nuclei, and green with Alexa 488 for DF), and Z projection (narrow images in the right and in the bottom of the main picture) show that DF remains in the cytoplasm displaying vesicular staining and does not enter into the nuclei, at least after 24 hours of incubation. Confocal images were taken using a Leica TCS SP5 microscope and a 63× oil immersion objective. Optical sections (z) were performed each 2 μm. Image analysis was performed using Fiji software (National Institutes of Health).
There was no staining when SK cells were incubated with Alexa 488 alone (Figure 3A) compared with the incubation of cells with DF labeled with Alexa 488 (Figure 3B). Exposure of HUVEC to DF (4 μg/mL, 4 hours) also resulted in the internalization of the drug (Figure 3C).
Specificity of DF labeling and DF interaction with ECs. (A) Confocal microscopy images show no green staining when SK cells (red staining with wheat germ agglutinin for membranes) were incubated only with Alexa 488 compared with (B) the incubation of SK cells with DF labeled with Alexa 488. (C) Exposure of HUVEC to DF (4 μg/mL) for 4 hours also resulted in the internalization of the drug. (D) Flow cytometry results show that SK and HUVEC follow the same DF interaction kinetics. PBMCs do not interact with DF. Confocal images were taken using a Leica TCS SP5 microscope and a 63× oil immersion objective. Image analysis was performed using Fiji software (National Institutes of Health).
Specificity of DF labeling and DF interaction with ECs. (A) Confocal microscopy images show no green staining when SK cells (red staining with wheat germ agglutinin for membranes) were incubated only with Alexa 488 compared with (B) the incubation of SK cells with DF labeled with Alexa 488. (C) Exposure of HUVEC to DF (4 μg/mL) for 4 hours also resulted in the internalization of the drug. (D) Flow cytometry results show that SK and HUVEC follow the same DF interaction kinetics. PBMCs do not interact with DF. Confocal images were taken using a Leica TCS SP5 microscope and a 63× oil immersion objective. Image analysis was performed using Fiji software (National Institutes of Health).
Flow cytometry experiments demonstrated that HUVECs showed almost identical uptake kinetics than SK cells. In contrast, PBMCs did not interact with DF even after 4 hours of exposure (Figure 3D).
DF is internalized by ECs through macropinocytic mechanisms
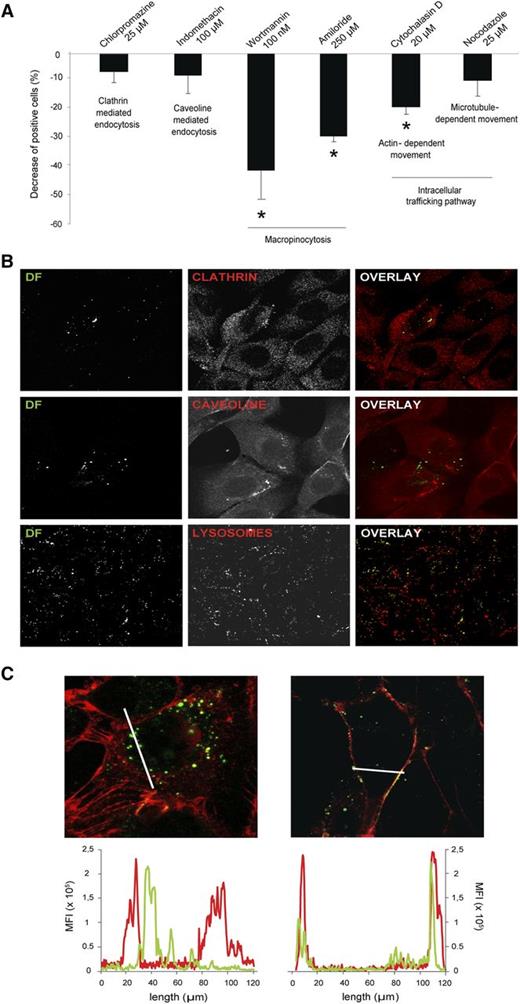
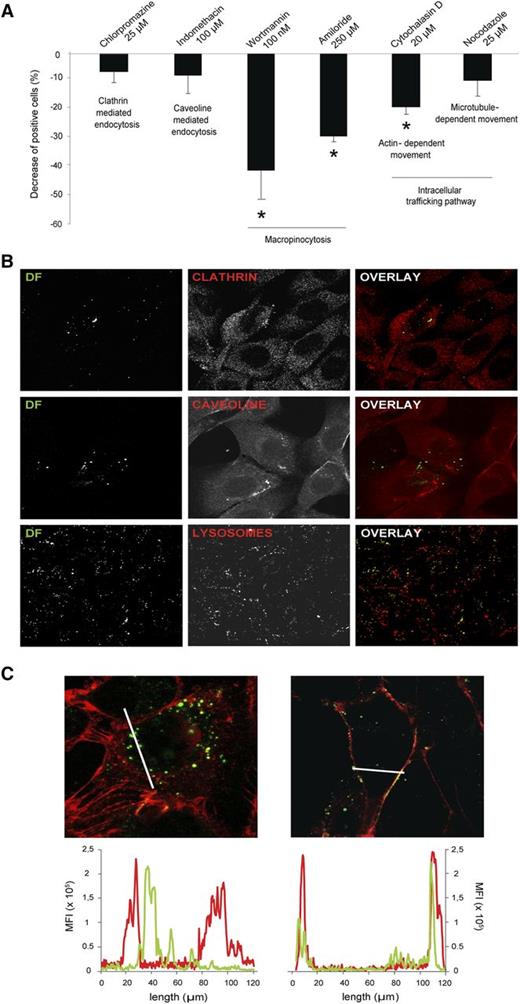
To explore several endocytic mechanisms potentially used by SK to internalize DF, assays in the presence of specific inhibitors were performed (Figure 4A). Considering that in control experiments, 100% of cells are able to incorporate DF, inhibition of clathrin- and caveola-mediated endocytosis by chlorpromazine and indiomethacine, respectively, resulted in a low and nonsignificant decrease in the percentage of positive cells for DF (decreases of 6% ± 3.7% and 7.1% ± 6.3%, respectively). Exposure of SK to the macropinocytosis inhibitors Wortmannin and amiloride resulted in a significant inhibition of the DF uptake (decreases of 39.7% ± 10% and 28% ± 2% vs control, respectively, P < .05, n = 6). The disruption of actin polymerization with cytochalasin D led to a decrease in the uptake of DF of 18.2% ± 2.4% (P < .05 vs control). However, blockade of microtubules with nocodazole did not result in a significant decrease in DF uptake (decrease of 9.1% ± 5.4%), suggesting that microtubules are not involved in the internalization of the drug.
DF is internalized by ECs through macropinocytic mechanisms. (A) Bar diagram shows the decrease in the uptake of DF by SK cells in the presence of endocytosis and vesicle-trafficking inhibitors. Data obtained from flow cytometry experiments are expressed as mean ± standard error of the mean, n = 6, being *P < .05 vs 100% of positive cells for DF in the absence of the inhibitors. (B) Confocal microscopy images correspond to the negative results of colocalization assays between DF (green, labeled with Alexa 488) and clathrin, caveoline, and lysosomes (first, second, and third lines, respectively). SK cells were incubated with DF for 15 minutes to evaluate colocalization with clathrin and caveoline, and for 6 hours to evaluate DF–lysosomal interaction. (C) Images to the left and right correspond to SK cells incubated with DF (green) in the absence or presence of Wortmannin (W), respectively (red staining with wheat germ agglutinin for membranes). Graphs above represent mean fluorescence intensity and follow the same distribution. DF staining inside the cells can be visualized in the absence of W (left image, left graphic). DF staining is attached to the membrane in the presence of W (right image, right graphic). Confocal images were taken using a Leica TCS SP5 microscope and a 63× oil immersion objective. Image analysis was performed using Fiji software (National Institutes of Health).
DF is internalized by ECs through macropinocytic mechanisms. (A) Bar diagram shows the decrease in the uptake of DF by SK cells in the presence of endocytosis and vesicle-trafficking inhibitors. Data obtained from flow cytometry experiments are expressed as mean ± standard error of the mean, n = 6, being *P < .05 vs 100% of positive cells for DF in the absence of the inhibitors. (B) Confocal microscopy images correspond to the negative results of colocalization assays between DF (green, labeled with Alexa 488) and clathrin, caveoline, and lysosomes (first, second, and third lines, respectively). SK cells were incubated with DF for 15 minutes to evaluate colocalization with clathrin and caveoline, and for 6 hours to evaluate DF–lysosomal interaction. (C) Images to the left and right correspond to SK cells incubated with DF (green) in the absence or presence of Wortmannin (W), respectively (red staining with wheat germ agglutinin for membranes). Graphs above represent mean fluorescence intensity and follow the same distribution. DF staining inside the cells can be visualized in the absence of W (left image, left graphic). DF staining is attached to the membrane in the presence of W (right image, right graphic). Confocal images were taken using a Leica TCS SP5 microscope and a 63× oil immersion objective. Image analysis was performed using Fiji software (National Institutes of Health).
Confocal analysis was also performed to confirm flow cytometry results. Exposure of SK to DF did not result in the colocalization of DF and clathrin, DF and caveoline (exposure for 30 minutes), or DF and lysosomes (exposure for 6 hours) (Figure 4B). DF appeared inside SK cells in the absence of Wortmannin and remained attached to the membrane in the presence of Wortmannin (Figure 4C, images left and right, respectively). These observations were confirmed by quantifying both the green and the red fluorescence, corresponding to DF and endothelial membrane staining, respectively (Figure 4C, graphics left and right, respectively). Therefore, macropinocytosis seems to be the mechanism for DF to enter the SK cells.
Inflammation of ECs is prevented by DF interaction with the cell membrane
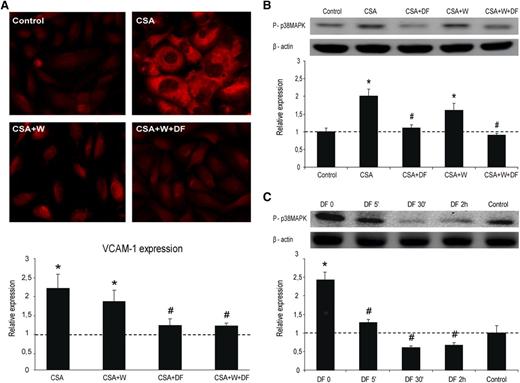
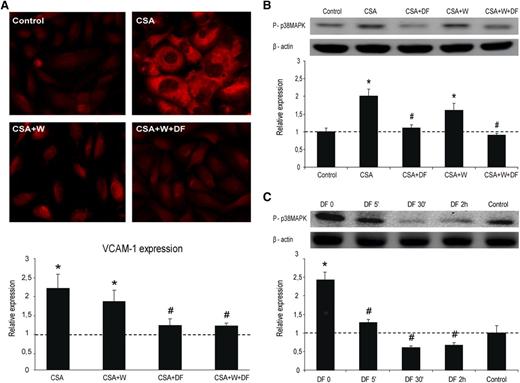
Increased expression of VCAM-1 was observed when SK were exposed to CSA alone (average fold increase of 2.2 ± 0.5 vs control; P < .05, n = 4). VCAM-1 expression was significantly reduced in SK exposed to CSA previously incubated with DF for 24 hours (average fold increase of 1.2 ± 0.2 vs control; P < .05 vs CSA, n = 4). Similar results were observed when cells were coincubated with Wortmaninn and DF (average fold increase of 1.2 ± 0.1 vs control; P < .05 vs CSA, n = 4) (Figure 5A). Presence of Wortmannin alone did not reduce the expression of VCAM-1 induced by CSA (1.8 ± 0.3 vs control; P < .05, n = 4) (Figure 5A).
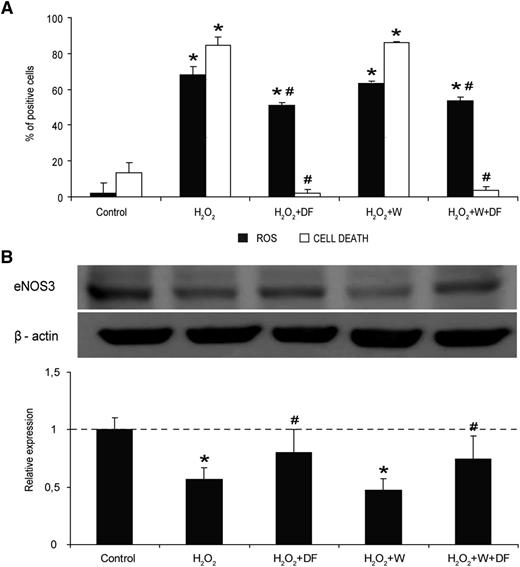
Inflammation of ECs is prevented by DF interaction with the cell membrane. (A) Micrographs show VCAM-1 expression on SK exposed to CSA (200 ng/mL, 24 h), without and with DF in the media and in the absence and presence of Wortmannin (W), as indicated. Bar diagrams represent levels of VCAM-1 expression on SK. (B) Activation of p38 MAPK in SK cells by CSA, without and with DF and in the absence and presence of W. Immunoblot image shows phosphorylated p38 MAPK, and the bar diagram represents the relative quantification (vs control). (C) Activation of p38 MAPK by CSA in SK cells previously incubated with DF for different time points. The immunoblot image shows phosphorylated p38 MAPK and the bar diagram represents the relative quantification (vs control). The dotted line represents the mean expression in control cells. All data correspond to relative expression, n = 4, being *P < .05 vs control and #P < .05 vs CSA.
Inflammation of ECs is prevented by DF interaction with the cell membrane. (A) Micrographs show VCAM-1 expression on SK exposed to CSA (200 ng/mL, 24 h), without and with DF in the media and in the absence and presence of Wortmannin (W), as indicated. Bar diagrams represent levels of VCAM-1 expression on SK. (B) Activation of p38 MAPK in SK cells by CSA, without and with DF and in the absence and presence of W. Immunoblot image shows phosphorylated p38 MAPK, and the bar diagram represents the relative quantification (vs control). (C) Activation of p38 MAPK by CSA in SK cells previously incubated with DF for different time points. The immunoblot image shows phosphorylated p38 MAPK and the bar diagram represents the relative quantification (vs control). The dotted line represents the mean expression in control cells. All data correspond to relative expression, n = 4, being *P < .05 vs control and #P < .05 vs CSA.
In western blot experiments, protein p38MAPK became phosphorylated in response to CSA (average fold increase of 2 ± 0.2 vs control; P < .05, n = 4), and the prophylactic treatment of cells with DF alone or in presence of Wortmannin was able to inhibit it (average fold increases of 1.1 ± 0.1 and 0.9 ± 0.1, respectively, vs control; P < .05 vs CSA) (Figure 5B). Wortmannin slightly reduced p38MAPK activation caused by CSA (average fold increase of 1.6 ± 0.2 vs control; P < .05 vs control). These results taken together suggest that the interaction of DF with the cell membrane is sufficient to provide an antiinflammatory effect on ECs.
In further experiments, SK were incubated with DF for different times (0, 5 minutes, 30 minutes, and 2 hours) and then exposed to CSA for 5 minutes (Figure 5C). CSA induced the phosphorylation of p38MAPK with respect to control (average fold increase of 2.4 ± 0.2 vs control; P < .05, n = 4). This activation was almost prevented by exposure to DF for 5 minutes (time at which DF is still attached to the membrane), at 30 minutes and after 2 hours (time at which DF is in the cytosol) (average fold increases of 1.3 ± 0.1, 0.6 ± 0.1, and 0.7 ± 0.1, respectively, vs control; P < .05 vs CSA, n = 4). These results reinforce our previous findings indicating that DF exhibits antiinflammatory effects on ECs by interacting with their membrane.
The antioxidant effect of DF on ECs is a result of its interaction with the cell membrane
By using flow cytometry, we evaluated at which cellular level DF performs its antioxidant effect on SK. Exposure of cells to 1 μM H2O2 induced a statistically significant increase in the production of intracellular ROS (% of positive cells increased from 2% ± 0.1% to 67.9% ± 5.5%; P < .05, n = 6) (Figure 6A, black bars). Presence of DF (500 μg/mL) prevented partially ROS generation (51.1% ± 5.4%; P < .05 vs control and H2O2 exposure), even in the presence of Wortmannin (53.8% ± 1.1%).
The antioxidant effect of DF on ECs is caused by its interaction with the cell membrane. (A) Flow cytometry experiments reveal that DF has an antioxidant effect in front of an oxidative stimuli (H2O2), even in the presence of Wortmannin (W). Black bars correspond to intracellular ROS in SK cells induced by incubation with 1 μM H2O2, expressed as percentage of positive cells. White bars correspond to percentage of dead cells after exposing SK to a high concentration of H2O2 (50 μM). (B) The immunoblot image shows changes in the presence of the protein eNOS3 in SK exposed to 1 μM H2O2, without and with DF in the media and in the absence and presence of Wortmannin (W), as indicated. The bar diagram represents relative presence of eNOS3 vs control. Data corresponds to n = 4, being *P < .05 vs control and #P < .05 vs H2O2.
The antioxidant effect of DF on ECs is caused by its interaction with the cell membrane. (A) Flow cytometry experiments reveal that DF has an antioxidant effect in front of an oxidative stimuli (H2O2), even in the presence of Wortmannin (W). Black bars correspond to intracellular ROS in SK cells induced by incubation with 1 μM H2O2, expressed as percentage of positive cells. White bars correspond to percentage of dead cells after exposing SK to a high concentration of H2O2 (50 μM). (B) The immunoblot image shows changes in the presence of the protein eNOS3 in SK exposed to 1 μM H2O2, without and with DF in the media and in the absence and presence of Wortmannin (W), as indicated. The bar diagram represents relative presence of eNOS3 vs control. Data corresponds to n = 4, being *P < .05 vs control and #P < .05 vs H2O2.
Moreover, the protective effect of DF in front of the cell death caused by the oxidative stress was evaluated by exposing SK to a high concentration of H2O2 (50 μM). The percentage of dead cells was 84.6% ± 4.7% with H2O2, decreasing to 2.2% ± 1.7% with DF (P < .05 vs H2O2, n = 6), a percentage even lower than that in controls (13.2% ± 5.8%). The protective effect of DF was also observed when cells were previously exposed to Wortmannin (3.8% ± 1.7%; P < .05 vs H2O2) (Figure 6A, white bars) and no effect could be attributed to Wortmannin itself (% of cell death 86.2 ± 0.3; P < .05 vs control).
Results from a protein array using microvascular ECs (data not shown) showed changes in the presence of eNOS3. We explored the potential involvement of eNOS3 in our experimental approach. Our results revealed that exposure of SK to H2O2 (1 μM) induced a decrease in the presence of eNOS3 (average fold decrease of 0.5 ± 0.1; P < .05 vs control, n = 4). Incubation with DF recovered eNOS3 presence in SK exposed to H2O2 in the absence and presence of Wortmannin (average fold decreases of 0.8 ± 0.1 and 0.7 ± 0.1, respectively, vs control; P < .05 vs H2O2) (Figure 6B).
Discussion
In the present study, we have investigated the mechanisms through which DF interacts with ECs. To our knowledge, this is the first comprehensive study describing the mechanistic details and kinetics by which DF interacts with ECs, demonstrating that it becomes attached to the external cell membrane and then becomes internalized by the cells. More interestingly, the present study provides direct evidence demonstrating that the interaction of DF with the cell membrane is sufficient to guarantee at least 2 of the several actions attributed to this drug on the endothelium, such as the antiinflammatory and antioxidant effects.
Vascular endothelium is a monolayer of cells that by its location is easily accessible to drugs that may target either this organ or the underlying tissues. Those drugs that target the endothelium should either bind to the EC surface or become internalized by these cells.26 Our results obtained from the flow cytometry approach, and further confirmed by confocal microscopy, demonstrated for the first time that exposure of ECs to DF induced a concentration, temperature, and time-dependent uptake of the drug by these cells.
Endocytosis of micro- and macromolecules occurs via diverse mechanisms that could be divided into 2 categories: phagocytosis and pinocytosis.27 Because the first one rarely occurs in ECs,27 we mainly focused on some of the mechanisms already described to participate in pinocytosis: clathrin- and caveolae-mediated uptake, and macropinocytosis. We further demonstrated that DF uptake by ECs predominantly involves a macropinocytic pathway, because its internalization by ECs was prevented by using amiloride, which inhibits the Na+/H+ exchange protein in the plasma membrane28 ; Wortmannin, a PI3K inhibitor; and by disrupting actin skeleton with cytochalasin D. Macropinocytosis has received special attention in the last decade as an entry route for genetic material and drug delivery,29,30 because this pathway provides some advantageous aspects such as the prevention of lysosomal degradation.24 In this regard, our results showed that DF does not seem to reach lysosomes in its traffic through the cytoplasm. After the formation of macropinosomes, their intracellular fate differs depending on the cell type.24,31 Apart from lysosomes, which do not seem to be the route for DF, another possible end point for these vesicles is to bind to the inner side of the cell membrane to release their content.32 However, our confocal microscopy studies demonstrate a tendency of DF to remain in cytoplasm or even locate at a perinuclear location after 24 hours of incubation.
Moreover, the role of adenosine receptors as potential mediators of DF uptake on the endothelium was also explored. In contrast to results from other authors,3,33 we did not find any significant effect of adenosine receptors’ blockade on EC–DF interaction. Although in our EC model we did not find any evidence of a specific receptor for DF, this drug seems to exhibit some specificity for ECs. More than a decade ago, Eissner et al21 demonstrated that DF did not interfere with the antileukemic effects of fludarabine over PBMCs. In addition, in the present study, we show that there is no interaction between DF and PBMCs, cells that also exhibit macropinocytic capacities.34
Previous studies from our group using ECs in culture characterized the antiinflammatory effect of DF in front of the endothelial activation and damage associated with autologous HCT6 and the deleterious effects of immunosuppressant drugs.7 To evaluate whether DF exerts its function after binding to the cell membrane or if it requires its internalization, we exposed DF-treated ECs to the immunosuppressant CSA in the absence and presence of Wortmannin. DF was able to inhibit the inflammatory reaction caused by CSA, decreasing the expression of VCAM-1 and the activation of p38MAPK, even when the macropinocytic entrance was inhibited, suggesting that DF may be acting at the membrane level.
Other effects ascribed to DF are the increase of the nitric oxide generation,35 the activation of NOS activity, and the reduction of oxidative stress.36 A reduction in hepatic nitric oxide levels has been shown to contribute to the development of SOS through the disruption of the sinusoidal integrity and subsequent disturbance of the sinusoides.37 To test the antioxidant effect of DF in our hepatic endothelial in vitro model, we used different concentrations of H2O2 as a chemical stimulator of the production of ROS. DF was very efficient at attenuating the generation of ROS, even in the presence of an entry blocker such as Wortmannin. Similar results were observed when excessive concentrations of H2O2 were used to induce cell death. In addition, ROS levels and nitric oxide bioavailability, both key factors of oxidative stress, are related to eNOS activity.38 ROS produced during endothelial dysfunction can promote eNOS uncoupling.39 In our in vitro model, the sole interaction of DF with cell membrane, even in the absence of significant internalization, was capable of restoring eNOS levels in front of oxidative stress.
Endothelial activation and damage are not only restricted to the HCT. Endothelial dysfunction is associated with a plethora of human disease conditions such as chronic renal failure,25,40 obesity,41 and sepsis,16,42 among others. Therefore, protection of the endothelium should be an initial step to prevent the development of vascular complications associated with some of the most prevalent diseases of the 21st century. DF seems to be an excellent candidate to explore as a potential EC protector. In addition, Mitsiades et al9 in an elegant study demonstrated that DF has anticancer properties, because it confers a modest tumor growth delay as a single agent but also enhances the antitumor activity of several chemotherapeutic agents. These actions seem to be caused by the inhibitory effect of DF on the interactions of tumor cells and nonmalignant cell compartments of the microenvironment, including stromal and ECs. Potential mechanisms that remain to be explored in the interaction between DF and different cell types could be the role of transporters that translocate nucleosides, single-stranded DNA-binding proteins,43 and charge dependent interactions as those reported for heparin.44
In conclusion, the present study provides the first evidence that DF interacts with the cell membrane of ECs of different origin and becomes internalized. The protective effect of DF over the endothelium is exerted through its interaction with the cell membrane. Because the single-stranded nucleotides that constitute this drug are also internalized by ECs, more research is needed to elucidate whether other effects of the drug are achieved in other cell localizations. Although far from patient daily concerns, our present findings constitute a potential major advance in the understanding of the mechanisms of action of DF and help to generate more confidence in the use of this drug in the clinical practice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Advanced Optical Microscopy Unit Staff (CCIT, University of Barcelona) for their support in confocal microscopy experiments, and the Primary Hemostasis Laboratory Group, especially Irene Lopez-Vílchez, for the contribution to the design of some experiments and technical support. They also acknowledge the collaboration of the staff of the Hospital de la Maternitat in Barcelona for providing the umbilical cords that made the some of the present results possible.
This research has been partially supported by Jazz Pharmaceuticals PLC/Gentium Inc.
Authorship
Contribution: M.P. designed and performed the experiments, analyzed the results, made the images, and wrote the manuscript; E.M. contributed to the design of experiments, cell cultures, confocal studies, and figure design; M.R. and G.E. reviewed the results and the manuscript; and E.C. and M.D.-R. designed and supervised the project and reviewed the results and the manuscript.
Conflict-of-interest disclosure: E.C. is a member of an expert panel and on the speakers’ bureau for Jazz Pharmaceuticals PLC/Gentium Inc. The remaining authors declare no competing financial interests.
Correspondence: Marta Palomo de Udaeta, Department of Hemotherapy and Hemostasis, Josep Carreras Leukaemia Research Institute, Hospital Clinic/University of Barcelona Campus, Villarroel 170, 08036 Barcelona, Spain; e-mail: mpalomo@carrerasresearch.org.