Key Points
Use of LMWH is associated with a lower risk of HIT and HITT compared with use of UFH.
The Avoid-Heparin Initiative resulted in a dramatic reduction in the burden of suspected HIT, adjudicated HIT, HITT, and associated costs.
Abstract
Heparin-induced thrombocytopenia (HIT) is an adverse drug reaction occurring in up to 5% of patients exposed to unfractionated heparin (UFH). We examined the impact of a hospital-wide strategy for avoiding heparin on the incidence of HIT, HIT with thrombosis (HITT), and HIT-related costs. The Avoid-Heparin Initiative, implemented at a tertiary care hospital in Toronto, Ontario, Canada, since 2006, involved replacing UFH with low-molecular-weight heparin (LMWH) for prophylactic and therapeutic indications. Consecutive cases with suspected HIT from 2003 through 2012 were reviewed. Rates of suspected HIT, adjudicated HIT, and HITT, along with HIT-related expenditures were compared in the pre-intervention (2003-2005) and the avoid-heparin (2007-2012) phases. The annual rate of suspected HIT decreased 42%, from 85.5 per 10 000 admissions in the pre-intervention phase to 49.0 per 10 000 admissions in the avoid-heparin phase (P < .001). The annual rate of patients with a positive HIT assay decreased 63% from 16.5 to 6.1 per 10 000 admissions (P < .001), adjudicated HIT decreased 79% from 10.7 to 2.2 per 10 000 admissions (P < .001), and HITT decreased 91% from 4.6 to 0.4 per 10 000 admissions (P < .001). Hospital HIT-related expenditures decreased by $266 938 per year in the avoid-heparin phase. To the best of our knowledge, this is the first study demonstrating the success and feasibility of a hospital-wide HIT prevention strategy.
Introduction
Heparin-induced thrombocytopenia (HIT) is a transient, limb- and life-threatening, immune-mediated adverse drug reaction in patients exposed to heparin. HIT is characterized by immunoglobulin G antibodies against platelet factor 4 (PF4)-heparin complexes which trigger a highly prothrombotic state through intravascular platelet aggregation, intense platelet activation, and excessive thrombin generation.1 The diagnosis of HIT is based on a significant decrease in the platelet count with or without venous or arterial thrombosis combined with serologic evidence of HIT antibodies in patients exposed to unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH).2-4 Treatment of HIT involves discontinuing all forms of heparin and administering an alternative nonheparin anticoagulant.4
HIT occurs in up to 5% of patients exposed to UFH, one of the drugs most commonly prescribed to hospital patients.5-7 The recognition and evaluation of suspected HIT is often delayed.4,6,8,9 Even with prompt cessation of heparin and implementation of a HIT-safe anticoagulant, thromboembolic complications occur in 20% to 50% of patients, and death or limb amputation occurs in approximately 5% to 10%.4,8,10-14 In addition to the significant disease burden, HIT is associated with substantial resource use. An economic analysis study from our center reported that the direct costs to the hospital for HIT were $456 787 over a 1-year period.15
Although improved surveillance and management may reduce the burden of HIT, comprehensive initiatives to prevent HIT are likely to be more effective in decreasing the morbidity, mortality, and costs associated with HIT. It is well established that LMWH is associated with a five- to 10-fold lower risk of HIT than UFH.16-21 Furthermore, thrombosis is less likely to occur when HIT is triggered by LMWH than by UFH.21 Therefore, reducing patient exposure to UFH and substituting LMWH for UFH may improve patient safety related to HIT. The aim of this quality improvement study was to evaluate the impact of an avoid-heparin intervention on the incidence of HIT, its clinical consequences, and associated costs over a 10-year period.
Methods
Study setting
In 2005, a multidisciplinary committee was created to develop strategies to reduce the burden of HIT at Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada. Sunnybrook is a tertiary care university-affiliated hospital with more than 450 adult acute care beds, a large cardiac surgery program, and a thromboembolism service that manages all cases of established HIT. The intervention selected was an institution-wide, avoid-heparin program that was implemented during 2006. The components of this program included (1) systematic replacement of most intravenous and subcutaneous UFH with subcutaneous LMWH in prophylactic or therapeutic doses (the remaining uses of UFH were for hemodialysis, intraoperative use for cardiovascular surgery, and for some patients with acute coronary syndrome), (2) replacement of heparinized saline in arterial and central venous lines with saline flushes, (3) modification of order sets to exclude UFH options, and (4) removal of UFH stores from most nursing units.
Most care providers were not aware that heparin was being replaced by LMWH as part of an avoid-heparin initiative, and none were aware that this practice change was being studied. There were also no efforts to educate staff about HIT nor were there any changes in the approach to its diagnosis.
Consecutive inpatients with a clinical suspicion of HIT who underwent enzyme-linked immunosorbent assay (ELISA) testing for PF4-heparin antibodies from January 1, 2003, to December 31, 2012, were identified through the Special Coagulation Laboratory database. A confirmatory serotonin release assay (SRA), which was performed at McMaster University, was ordered at the discretion of the patient’s attending physician or consultant service. Electronic and paper medical records were reviewed for demographic and clinical data in all patients with a positive HIT ELISA. The incidence and complications of HIT and associated costs were compared in the pre-intervention phase (2003-2005) and the avoid-heparin phase (2007-2012). Because the avoid-heparin intervention was implemented over the year 2006, cases during this year were excluded from all comparisons. This study was approved by the research ethics board of Sunnybrook Health Sciences Centre.
Case definitions
Explicit definitions of the various study groups were established a priori (supplemental Table 1, available on the Blood Web site). Suspected HIT was defined as a clinical suspicion of HIT with a HIT ELISA performed. A positive HIT ELISA was defined as a HIT ELISA optical density (OD) ≥0.4. Patients with a positive HIT ELISA who were excluded were those who underwent follow-up testing for a previously positive HIT assay, those whose heparin exposure occurred exclusively as an outpatient or at another hospital, and those who had no documented heparin exposure despite an extensive search. The following data were abstracted: demographic information, admitting service, duration and type of heparin exposures, date of suspected HIT, presence of HIT-related complications, and length of hospital stay.
All patients with a positive HIT ELISA were adjudicated by the investigators who used information available in the medical record. Adjudicated HIT was defined as suspected HIT with (1) positive SRA or (2) positive HIT ELISA, SRA not done, and diagnosed and treated as HIT by the thromboembolism service at the time of the suspected HIT and confirmed at the independent adjudication review by using standardized criteria outlined in supplemental Table 1. HIT negative was defined as suspected HIT with (1) negative HIT ELISA, (2) negative SRA, or (3) positive HIT ELISA, SRA not done, but not diagnosed with or treated as HIT by the thromboembolism service at the time of suspected HIT and also classified as HIT negative during adjudication review.
Cases were labeled HIT uncertain if the HIT ELISA was positive but the diagnosis could not be confirmed or ruled out at adjudication. In the small number of cases with a positive HIT ELISA that were not seen by the thromboembolism service or when the adjudication process yielded a different HIT status for a patient than that made at the time of clinical suspicion, the case was re-reviewed, and consensus among investigators was used based on the diagnostic criteria in supplemental Table 1. HIT with thrombosis (HITT) was defined as HIT positive with proven venous and/or arterial thrombosis less than 7 days before or up to 30 days after the date of suspected HIT. For thrombotic events that occurred less than 7 days before the date of suspected HIT, each case was carefully reviewed to determine the probable sequence of events. Patients with thromboembolism prior to heparin exposure who subsequently developed HIT were not labeled as HITT. Major bleeding was defined as overt bleeding in a patient who was receiving a HIT-safe anticoagulant and who met at least one of the following criteria: required 2 or more units of packed red blood cells, had prolonged hospital admission, had fatal or life-threatening bleeding at a critical site such as intracranial or retroperitoneal, or had bleeding that required an intervention such as endoscopic or endovascular treatment or surgery.22
Anti-PF4-heparin HIT ELISA
Throughout the study period, the Special Coagulation Laboratory detected polyspecific PF4-heparin antibodies by using the Genetic Testing Institute PF4 ELISA (GTI Diagnostics, Waukesha, WI).23 An OD value of ≥0.4 was used as the threshold for a positive test as suggested by the manufacturer and locally validated by the laboratory.
Outcomes
The rates of suspected HIT, positive HIT ELISA, adjudicated HIT, and HITT in the pre-intervention and the avoid-heparin phases were compared. Annual rates per 10 000 admissions were determined by using admission data from the Health Data Resources Department. The additional costs for suspected HIT, adjudicated HIT, and HITT were based on a published cost-analysis done at our institution and the observed events in this study.15 HIT-related costs in the pre-intervention phase were compared with those in the avoid-heparin phase.
Statistical analyses
Statistical analyses were performed with SPSS 16.0 (SPSS, Chicago, IL). Continuous variables were summarized as medians with interquartile ranges (IQRs) because of non-normal distributions. The Mann-Whitney U test was used to detect differences in continuous variables, and the Pearson χ2 test or Fisher’s exact test was used to detect differences between categorical variables. All tests were two sided, and P < .05 was accepted as statistically significant.
Results
Suspected HIT, positive HIT ELISA, adjudicated HIT, and HITT
From 2003 to 2012, there were 1118 cases of suspected HIT. Among these, 175 patients (16%) had a positive HIT ELISA. An additional 16 patients with a positive HIT ELISA were excluded because they received heparin exclusively as an outpatient or at another institution (10), there was no documented heparin exposure (2), the HIT assay was ordered for a clinical trial (2), or it was performed in error (1), or for previous HIT (1). Among the 175 patients with a positive HIT ELISA, 89 (51%) were adjudicated HIT positive, 84 (48%) HIT negative and, in two cases, the HIT status remained uncertain after adjudication. An SRA was performed in 40% of patients (70 of 175) with a positive HIT ELISA (supplemental Table 2). Among the 84 patients adjudicated as HIT negative, 46 (55%) had a negative SRA, 37 (44%) did not have an SRA performed, 1 had an SRA reported as equivocal, and none had a positive SRA. Among the 37 patients adjudicated HIT negative who did not have an SRA performed, only 5 had a HIT ELISA OD >1.0. A HIT ELISA OD ≥1.0 was observed in 75 patients (84%) with adjudicated HIT compared with 16 patients (19%) with a positive HIT ELISA who were adjudicated HIT negative (P < .001). Over the 10-year period, 31 patients (35%) with adjudicated HIT developed HITT. In 10 of these patients, the thromboembolic event occurred 1 to 5 days before the diagnosis of HIT and 4 were diagnosed with thromboembolism and HIT on the same day.
Comparison of the pre- and postintervention periods
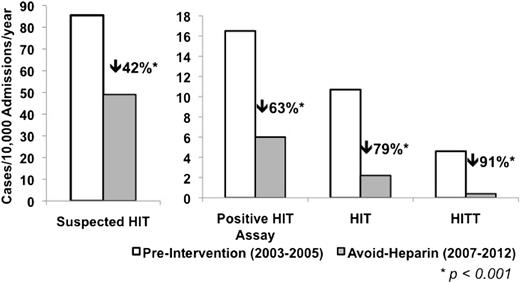
There were 424 patients with suspected HIT in the pre-intervention phase and 576 patients with suspected HIT in the avoid-heparin phase. The annual incidence of suspected HIT cases per 10 000 admissions decreased from 85.5 in the pre-intervention phase to 49.0 in the avoid-heparin phase (relative risk reduction [RRR], 41.7%; P < .001; Figure 1). The annual incidence of patients with a positive HIT ELISA decreased from 16.5 to 6.1 per 10 000 admissions in the pre-intervention and avoid-heparin phases, respectively (RRR, 62.9%; P < .001; Figure 1), and corresponding rates of patients with a HIT ELISA ≥1.0 decreased from 10.1 to 2.5 per 10 000 admissions (RRR, 75.1%; P < .001). The annual incidence of adjudicated HIT cases decreased from 10.7 to 2.2 per 10 000 admissions in the pre-intervention and avoid-heparin phase (RRR, 79.0%; P < .001), and HITT decreased from 4.6 to 0.4 per 10 000 admissions (RRR, 90.7%; P < .001; Figure 1). The annual rates of HIT and HITT over the study period are shown in Figure 2.
Annual incidence of suspected HIT, positive HIT assay, adjudicated HIT, and HITT per 10 000 admissions, 2003-2005 and 2007-2012. *P < .001 for each comparison.
Annual incidence of suspected HIT, positive HIT assay, adjudicated HIT, and HITT per 10 000 admissions, 2003-2005 and 2007-2012. *P < .001 for each comparison.
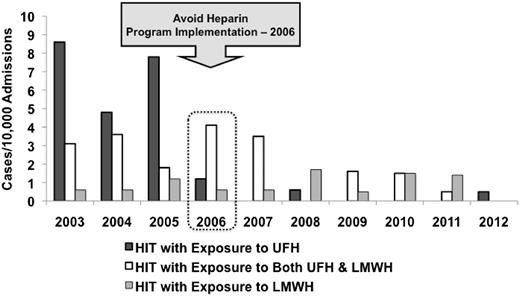
Demographic and clinical characteristics of HIT-positive patients in the pre-intervention phase (n = 53) and the avoid-heparin phase (n = 26) were similar (Table 1). Approximately 60% of patients with HIT in both phases were admitted under the cardiovascular surgery service. The durations of UFH and/or LMWH exposure and median lengths of hospital stay were similar between the groups. The median HIT ELISA OD among patients with HIT was significantly higher in the avoid-heparin phase (2.79; IQR, 1.76-2.89) compared with the pre-intervention phase (2.07; IQR, 1.21-2.40; P = .001). Whereas the overall use of LMWH increased fourfold (based on doses purchased) over the study period, the annual rate of HIT associated with LMWH remained constant at 0.9 cases per 10 000 admissions in the pre-intervention and avoid-heparin phases (P = .78; Figure 3). There were no significant differences in patient age, sex, duration of UFH or LMWH exposure, or length of hospital stay among patients with HIT exposed to UFH compared with LMWH (supplemental Table 3). HIT was reduced 77% in cardiovascular surgery, 77% in other surgery, 75% in cardiology patients, and 62% in medical patients (supplemental Figure 1).
Incidence of UFH- and LMWH-associated HIT cases per 10 000 admissions per year, 2003-2012.P < .001 for UFH-associated HIT time trends and P = .78 for LMWH-associated HIT time trends.
Incidence of UFH- and LMWH-associated HIT cases per 10 000 admissions per year, 2003-2012.P < .001 for UFH-associated HIT time trends and P = .78 for LMWH-associated HIT time trends.
Comparison of HIT with and without thrombosis
Patients with HIT in the pre-intervention phase more frequently developed HITT (43%) than patients with HIT in the avoid-heparin phase (19%; P = .035; Table 2). The most frequently observed HIT complication in both phases was venous thromboembolism. The median length of stay from date of suspected HIT to discharge among patients with HITT was 22 days (IQR, 13-44 days) compared with 8 days (IQR, 6-14 days) for patients with HIT without thrombosis (P < .001). Over the entire study period, the HIT ELISA OD values were similar among patients with HIT without thrombosis (median OD, 1.96; IQR, 1.24-2.74) and those with HITT (median OD, 2.34; IQR, 1.86-2.70; P = .157).
Patient age, sex, admitting service, exposure to LMWH only, duration of heparin exposure, and alternative anticoagulant use were similar among patients with HITT and those with HIT without thrombosis (supplemental Table 4).
HIT-related treatment and costs
The mean number of suspected HIT cases per year decreased from 141.3 in the pre-intervention phase to 96.0 in the avoid-heparin phase (supplemental Table 5). The mean number of patients per year with a positive HIT ELISA, adjudicated HIT, and HITT per year decreased from 27.3 to 11.8, 17.7 to 4.3, and 7.7 to 0.8, respectively, in the pre-intervention and avoid-heparin phases. HIT was treated with lepirudin and danaparoid more frequently in the pre-intervention phase whereas fondaparinux and argatroban were used more frequently in the avoid-heparin phase (supplemental Table 6). On the basis of a published study from our institution,15 the average estimated costs of HIT care per year were reduced by $266 938, from $322 321 in the pre-intervention phase to $55 383 in the avoid-heparin phase, using 2007 Canadian dollars (Table 3).
Discussion
Following the implementation of a hospital-wide quality improvement program based on replacing UFH with LMWH, we observed a dramatic reduction in the burden of HIT with a 42% decrease in suspected HIT, 63% decrease in patients with positive HIT ELISA, 79% decrease in adjudicated HIT, 91% decrease in HITT, and 83% decrease in HIT-related costs of care. Although the greatest overall impact of the program was in cardiac surgery, the HIT burden was also reduced in other surgical and medical patients. The heparin avoidance strategy that we used was not complex or costly and would be feasible in other centers.
A lower risk of HIT and its thrombotic complications among patients exposed to LMWH compared with UFH has been previously demonstrated.7,16-21 LMWH is thought to induce a less robust antibody response when complexed to PF4 than observed with UFH-PF4 because of stoichiometric differences of heparin-PF4 complexes.24 Despite the fourfold increase in use of LMWH over the study period, the incidence of HIT in patients who received LMWH was low and remained stable over the 10-year study period. Others have also demonstrated stable rates of HIT despite substantial increases in LMWH use.7,25 Our results expand on observations from studies with limited target groups and focused interventions. Replacement of UFH with LMWH in orthopedic surgery has been shown to reduce both venous thromboembolism and HIT.26
The literature has emphasized early recognition and treatment of HIT, but its prevention has been largely overlooked.27 Guidelines recommend platelet count monitoring for patients receiving heparin who have a HIT risk greater than 1%.4 However, adherence to platelet count monitoring, testing for HIT antibodies if thrombocytopenia develops, and switching to HIT-safe anticoagulation when HIT is suspected is challenging, resource intensive, and may not reduce the adverse consequences of HIT.6,9,28-30 Previous studies have demonstrated that more than half the thrombotic events in HIT occur after the cessation of heparin without additional treatment of HIT.8,11 In our study, we observed that more than half the HITT cases (17 of 31) had the thromboembolic event diagnosed after the diagnosis of HIT and while they were receiving HIT-safe anticoagulation, which emphasizes not only the need for early recognition and treatment but also the need to prevent HIT. We observed that patients with HIT in the avoid-heparin phase were 2 times less likely to develop HITT. A previous study from our center demonstrated that the incremental hospital cost of suspected and confirmed cases of HIT was $456 787 over a 1-year period, and 90% of these costs were attributed to HITT.15 Together, these findings suggest that a hospital-wide, avoid-heparin program leads to a substantial reduction in the morbidity, mortality, and institutional costs associated with HIT.
Consistent with published literature, the positive predictive value of the HIT assay was 51% (89 of 175) over the entire study period.31,32 Among the 84 patients with a HIT ELISA OD between 0.4 and 1.0, only 17% were found to have HIT. False-positive HIT assays were found with OD values as high as 2.4, emphasizing that HIT cannot be diagnosed by laboratory evidence alone.4,33 The median HIT ELISA OD in patients with HIT was higher in the avoid-heparin phase than the pre-intervention phase (2.79 vs 2.07, respectively; Table 1). Because the likelihood of true HIT increases with increasing OD, this could suggest that there were more false-positive diagnoses in the pre-intervention phase. Recently, Chan et al34 reported that the HIT ELISA OD cutoff could be increased from 0.4 to 1.00 to improve the positive predictive value without losing sensitivity.
This study has limitations that warrant consideration. It was conducted at a single tertiary care hospital whose HIT-related practices may differ from those of other centers. Because there is no reference standard for the diagnosis of HIT and because only 40% of patients with a positive HIT ELISA had an SRA, we cannot determine the accuracy of our case allocation in all patients. However, the observations in this study were based on a review of all cases with suspected HIT over a 10-year period and therefore reflect routine clinical practice. We attempted to minimize potential bias in adjudication of HIT by establishing case definitions a priori. In only 5 cases did the final case allocation after adjudication differ from that made by the clinical team at the time of suspected HIT. Although this study’s findings are based on a prospective quality improvement project, patient data were abstracted, and cases were adjudicated retrospectively. This could have led to possible bias in case definition by the investigators. We were unable to calculate the 4Ts score for patients with suspected HIT retrospectively.35,36 However, patient characteristics in the pre-intervention and avoid-heparin phases were similar, and we were unable to identify any other factors that could have accounted for the dramatic reductions in suspected HIT, adjudicated HIT, and HITT during the study period. The long duration of observation showing a sudden and sustained reduction in HIT after implementation of the avoid-heparin strategy was designed to demonstrate that this was not a random observation. Finally, we were unable to determine hospital-wide, patient-level data on exposures to UFH and LMWH; however, LMWH use increased more than fourfold on the basis of overall changes in drug use over the study period. Clearly, the cost reductions we observed are specific to our center and will differ in other centers, depending on overall HIT burden and the local costs of investigating and treating HIT.
Our findings cannot be explained by an overall reduction in the number of admissions or length of stay over the study period. In fact, total patient admissions increased 21% whereas the mean length of stay decreased only 9% over the study period. The decision to test each patient was at the discretion of the patient’s clinical service and was not influenced by the investigators or the thromboembolism team. Moreover, the greater reduction in cases of HIT (79%) and HITT (91%) than in suspected HIT (42%) suggests a decrease in the actual disease burden rather than a biased increase in diagnostic threshold (Figure 1). The temporal pattern of this study’s findings show that the reduction in HIT was not gradual but occurred in 2006 and was maintained beyond the intervention year (Figure 2), suggesting that it is unlikely that secular trends in decreased use of UFH could explain these findings. The study’s observations can also not be accounted for by use of direct oral anticoagulants because rivaroxaban was approved only for hip and knee arthroplasty thromboprophylaxis in 2009 and dabigatran was approved for atrial fibrillation in 2011.
In conclusion, the introduction of a hospital-wide, avoid-heparin program led to a dramatic decrease in the burden of suspected HIT, diagnosed HIT, and HITT, as well as in the costs of HIT care. To the best of our knowledge, this is the first study demonstrating the success of a HIT prevention strategy. Our findings suggest that a highly feasible heparin avoidance intervention can improve patient safety and reduce hospital costs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; W.G., C.B., A.D., and R.S. developed the study concept and design; W.G., K.E.M., J.M., A.D., C.B., and P.R. acquired the data; W.G., K.E.M., J.M., A.D., C.B., and R.S. analyzed and interpreted the data; W.G., K.E.M., J.M., A.D., and C.B. drafted the manuscript; all authors critically revised the manuscript; and W.G., J.M., A.D., C.B., and R.S. provided administrative, technical, and material support.
Conflict-of-interest disclosure: J.M. received financial support from Bayer Healthcare for an institutional fellowship. A.D. was supported by a hospital account that had contributions from the Canadian Patient Safety Institute and Sanofi, consulted for Leo Pharma and Sanofi, served on an advisory board for Sanofi, and received support for educational activities from Bayer Healthcare, Leo Pharma, Pfizer, and Sanofi. C.B. received support for educational activities from AstraZeneca and Bayer Healthcare, consulted for AstraZeneca, Bayer Healthcare, Bristol-Myers Squibb, and Pfizer, and served on an advisory board for AstraZeneca, Bristol-Myers Squibb, and Pfizer. P.R. was supported by an unrestricted educational grant for a studentship from Bayer Healthcare. R.S. was supported by a grant from Boehringer-Ingelheim, consulted for Instrumentation Laboratories, and was supported by Bristol-Myers Squibb and Pfizer for educational activities. W.G. received partial salary support from a hospital account that had contributions from the Canadian Patient Safety Institute and Sanofi, consulted for Bayer Healthcare, Boehringer-Ingelheim, Leo Pharma, Pfizer, Janssen, Bristol-Myers Squibb, and Sanofi, and received support for educational activities from Bayer Healthcare, Boehringer-Ingelheim, Leo Pharma, Pfizer, GlaxoSmithKline, and Sanofi. The remaining author declares no competing financial interests.
Correspondence: William Geerts, Sunnybrook Health Sciences Centre, University of Toronto, Room D674, 2075 Bayview Ave, Toronto, ON, Canada M4N 3M5; e-mail: william.geerts@sunnybrook.ca.
References
Author notes
K.E.M., J.M., A.D., C.B., and W.G. contributed equally to this work.