Key Points
Acute myocardial infarction and ischemic stroke risk was 3 times higher during days 0 to 1 following IVIg treatment in patients with secondary hypogammaglobulinemia.
In patients treated with IVIg for 1 year, the estimated increase in the absolute risk of a severe thromboembolic event was ∼1%.
Abstract
In patients with hypogammaglobulinemia secondary to chronic lymphocytic leukemia (CLL) or multiple myeloma (MM), intravenous immune globulin (IVIg) may be administered to reduce the risk of infection. Since 2013, IVIg products have carried a boxed safety warning about the risk of thromboembolic events (TEEs), with TEEs reported in 0.5% to 15% of patients treated with IVIg. In this retrospective cohort study of older patients with CLL or MM identified from the Surveillance, Epidemiology, and End Results-Medicare Linked Database, we assessed rates of clinically serious TEEs in 2724 new users of IVIg and a propensity-matched comparison group of 8035 nonusers. For the primary end point, arterial TEE, we observed a transient increased risk of TEE during the day of an IVIg infusion and the day afterward (hazard ration = 3.40; 95% confidence interval [CI]: 1.25, 9.25); this risk declined over the remainder of the 30-day treatment cycle. When considered in terms of absolute risk averaged over a 1-year treatment period, the increase in risk attributable to IVIg was estimated to be 0.7% (95% CI: −0.2%, 2.0%) compared with a baseline risk of 1.8% for the arterial TEE end point. A statistically nonsignificant risk increase of 0.3% (95% CI: −0.4%, 1.5%) compared with a baseline risk of 1.1% was observed for the venous TEE end point. Further research is needed to establish the generalizability of these results to patients receiving higher doses of IVIg for other indications.
Introduction
Intravenous immune globulin (IVIg) is a purified plasma fraction of nonspecific or polyvalent immunoglobulin G (IgG) derived from large donor pools; it is used for a variety of immunologic indications.1 In patients with chronic lymphocytic leukemia (CLL, also referred to as small lymphocytic lymphoma) or multiple myeloma (MM) who are at high risk of infection due to secondary hypogammaglobulinemia, IVIg may be administered every 3 to 4 weeks to reduce the risk of serious infection.2-4
In 2013, the US Food and Drug Administration (FDA) mandated that IVIg products include a prominent boxed warning about the risk of adverse thromboembolic events (TEEs),5-7 raising questions among patients and clinicians about the risk-benefit ratio of IVIg. Since the 1980s, there have been reports of serious IVIg-associated adverse TEEs, including acute myocardial infarction (AMI), ischemic stroke, and venous thromboembolism.8-10 Between 2006 and 2010, 209 TEEs associated with immune globulin treatment were reported via the FDA Adverse Events Reporting System.10
IVIg-associated TEE reports led to the voluntary withdrawals of the IVIg products Octagam from the US market in 2010 and Omr-IgG-am from the Israeli market in 2011.11,12 In response to these events, the FDA collaborated with academic researchers on laboratory and epidemiologic assessments of the thrombogenic potential of immune globulin products. In the epidemiologic study based on data from a large insurance claims database, it was found that ∼1% of IVIg-treated patients had a same-day TEE diagnosis recorded, with higher risks observed among patients with preexisting risk factors (eg, advanced age, hypercoagulable state), and those who received certain brands of IVIg.13
Several pathophysiologic mechanisms by which IVIg may increase thrombotic risk have been proposed. Administration of IVIg measurably increases blood viscosity,14-16 perhaps by promoting erythrocyte aggregation,17 which could contribute to localized blood stasis and thrombosis. It has also been suggested that exogenous IgG may promote platelet activation18 and arterial vasospasm.19 Laboratory assessments and evidence reviewed at a 2011 FDA Public Workshop implicated plasma constituents, such as coagulation factor XIa, copurified with IgG or otherwise not wholly removed in some IVIg manufacturing processes.11,13,20-23
Prior studies have provided varying estimates of the frequency of TEEs in those receiving IVIg, with reports of TEEs occurring in 0.5% to 15% of patients treated with IVIg.13,14,24-32 However, the extent to which these events are attributable to the IVIg itself rather than other risk factors is unclear, as is the risk associated with low doses of IVIg for immunodeficiency. In this retrospective cohort study, we assessed rates of clinically serious arterial and venous TEEs in older patients with CLL or MM who initiated IVIg therapy. Event rates in new users were compared with those in a propensity-matched comparison group of nonusers. Based on prior reports9,10,13 and potential biological mechanisms, we hypothesized that the prothrombotic effects of IVIg would most likely be acute but could last as long as 1 month after an IVIg infusion.
Methods
Data source
The study data were from the Surveillance, Epidemiology and End Results (SEER) cancer registries (1976-2009 for CLL patients; 1984-2009 for MM patients) linked to Medicare claims and enrollment data (1991-2010).33 Within SEER registry areas, ∼93% of patients age ≥65 years diagnosed with cancer have been linked to Medicare claims data. Participating registries collect data for all cancer patients diagnosed within their defined geographic area. Registry data include month and year of diagnosis, age at diagnosis, race, tumor stage, and histology. Medicare files from the Centers for Medicare and Medicaid Services include demographic and enrollment information, date of death, and all bills submitted for inpatient hospital care, outpatient hospital care, physician services, and prescription fills.
This study was approved by the University of Iowa Institutional Review Board. SEER-Medicare staff reviewed the manuscript to ensure that it complies with SEER-Medicare reporting guidelines for protecting patient confidentiality.
Cohort definition and follow-up
During the study period (1992-2010), new IVIg users and nonuser controls were identified from the population of SEER-Medicare patients who had been diagnosed with CLL or MM. Patients were eligible for entry into the study cohort if they were ≥66 years old, had been diagnosed with CLL within 16 years or MM within 8 years, and had maintained traditional fee-for-service Medicare Parts A and B coverage for the past year. Because we restricted to individuals eligible for Medicare coverage by virtue of age, age 66 was the earliest point at which they would have the required 1-year lookback period for the assessment of baseline health conditions and health care utilization. The years-since-diagnosis eligibility requirement was informed by the typical prognosis for patients diagnosed with CLL or MM.34,35
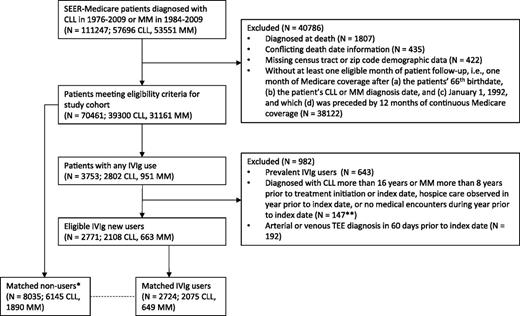
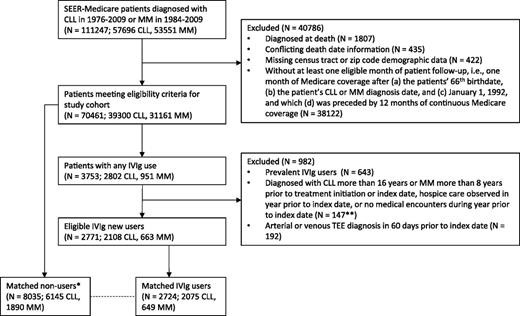
We also required evidence of health care utilization (≥1 Medicare inpatient, outpatient, or provider claim) and no hospice care within the prior year. Patients who used IVIg prior to reaching eligibility for study inclusion were classified as prevalent users and excluded. An account of all study inclusion criteria and the number of patients excluded at each step are provided in Figure 1.
Application of study inclusion criteria to select matched cohort of eligible IVIg users and nonusers diagnosed with CLL or MM. *In identifying potential matches for an IVIg user, all exclusion criteria applied to IVIg users were similarly applied to the pool of potential nonuser matches on the IVIg user’s treatment initiation date (index date). **Counts for these 3 exclusion criteria were combined due to SEER-Medicare restrictions on publishing small cell counts.
Application of study inclusion criteria to select matched cohort of eligible IVIg users and nonusers diagnosed with CLL or MM. *In identifying potential matches for an IVIg user, all exclusion criteria applied to IVIg users were similarly applied to the pool of potential nonuser matches on the IVIg user’s treatment initiation date (index date). **Counts for these 3 exclusion criteria were combined due to SEER-Medicare restrictions on publishing small cell counts.
For each patient who met eligibility criteria and initiated IVIg treatment during the study period, 1 to 3 no-users with the same (1) type of cancer (CLL or MM) and (2) Medicare Part D utilization status during the 1-year lookback period (yes or no) were matched on time-dependent propensity scores (see Statistical methods section). As shown in supplemental Figure 1 available on the Blood Web site, follow-up for each set of matched patients began on the IVIg user’s treatment initiation date and continued until the first of the following: occurrence of an acute TEE, completion of 1 year of follow-up, death, end of the study period, treatment discontinuation by an IVIg user (>90 days with no IVIg), initiation of IVIg by a nonuser, use of a subcutaneous or intramuscular immune globulin product, or termination of traditional fee-for-service Medicare coverage. To ensure that the IVIg user and nonuser groups had similar follow-up duration and remained balanced over the 1-year follow-up period, we also censored a matched set when follow-up ended for the IVIg user or all nonusers within that set.
Exposure
Receipt of IVIg therapy was determined from patients’ Medicare Parts A, B, and D claims. Specific codes used to define IVIg exposure are listed in supplemental Table 1. To assess the temporal relationship between IVIg use and TEE risk, for each day of follow-up for IVIg users, we determined how recently the patient had received IVIg. Exposure recency was classified as 0 to 1 days (any day on which IVIg was administered and the day thereafter), 2 to 15 days, 16 to 30 days, or 31 to 90 days after IVIg. We also estimated the average risk of TEE over the day 0 to 30 exposure period (which corresponds to the maximum interval between IVIg treatments for a typical immunodeficiency patient) by combining exposure categories.
When available, line-item and procedure dates on the claims were used to determine the specific dates on which IVIg was administered; otherwise, the claim start and end dates were used. For the doses typically given for immunodeficiency, we would expect an IVIg treatment episode to last 1 to 2 days. When the date range associated with an IVIg treatment record was >2 days, we classified patients’ IVIg exposure status as indeterminate from the start of that date range until a date-specific treatment record was observed, due to uncertainty about the exact dates on which IVIg was administered during those periods (supplemental Table 2).
Outcomes
End points were defined using principal diagnosis codes recorded on hospital inpatient claims. The primary outcome was the occurrence of serious arterial TEE, defined as hospitalization for AMI or ischemic stroke. Secondary outcomes included venous TEE (hospitalization for deep vein thrombosis [DVT] or pulmonary embolism [PE]) and a composite TEE end point (hospitalization for AMI, ischemic stroke, DVT, or PE). We considered the venous TEE end point to be secondary because of the greater potential for confounding and surveillance bias, because hospitalization and immobility are strong drivers of venous TEE risk,36 and patients may not seek immediate medical attention for less severe signs and symptoms of venous TEE. Diagnosis codes used to define the outcomes (supplemental Table 3) were chosen to maximize the end point definitions’ positive predictive value (PPV) for true acute TEEs, as reported by prior chart validation studies.37-39
Because TEE diagnoses recorded in claims data may reflect continuing care for a recent event rather than the occurrence of an acute TEE, we excluded patients with an acute TEE diagnosis recorded in the 60 days prior to the start of follow-up. The exclusion was based on the presence of an inpatient diagnosis code for AMI or ischemic stroke or an inpatient or outpatient diagnosis of DVT or PE, as venous thromboembolism may be managed on an outpatient basis.
Because our end point definitions were restricted to principal inpatient diagnoses reflecting the primary reason for a patient’s hospital admission, the date of admission was assumed to be the date of the event onset. If a patient was admitted to the hospital for another reason during the course of follow-up, we excluded person-time from day 2 through discharge of the hospital stay from the analysis, because a TEE that arose during a hospital stay would appear as a secondary position diagnosis under standard coding practices40 and the precise date of onset would be unknown.
Covariates
We determined patients’ demographic characteristics and time since diagnosis with CLL or MM from SEER Registry and Medicare enrollment data. Patients’ health care utilization and health conditions associated with arterial TEE risk, venous TEE risk, degree of cancer progression, and the presence of an immunodeficiency indication for IVIg treatment were assessed using claims data from the 365 days prior to entry into the study cohort. These patient characteristics were used as predictors of patients’ relative propensity for initiating IVIg treatment, as described in the Statistical methods section. Health conditions included in the propensity model and the codes used to define them are listed in supplemental Tables 4 and 5.
As exploratory analyses, we examined whether the association between IVIg treatment and TEE risk was modified by the following factors: calendar year, age, type of cancer (CLL or MM), history of clinical atherosclerotic cardiovascular disease (coronary heart disease, cerebrovascular disease, and/or peripheral vascular disease), history of prior venous TEE, hospitalization within the prior year, Part D status, days since IVIg initiation, and IVIg product brand (as identified in the administrative billing data). To test for interaction, we added each of these covariates as a main effect and interaction term to the model and tested whether the composite TEE hazard ratio for days 0 to 30 after IVIg differed across levels of the covariate.
Statistical methods
New IVIg users were individually matched to 1 to 3 nonusers with the same type of cancer (CLL or MM) and Part D utilization status (yes/no) on time-dependent propensity scores (TDPSs) derived from Cox regression models.41 Among CLL and MM patients, the length of time between initial cancer diagnosis and the emergence of a humoral immunodeficiency constituting an indication for IVIg use is variable. By matching calendar date and TDPSs, nonusers can be selected for study inclusion on the date that they are most comparable to an IVIg new user on measurable indicators of thrombotic risk, cancer progression, and comorbidity burden observed during the prior year. In principle, TDPS matching will yield a cohort of IVIg users and nonusers who are balanced on these factors at the start of patient follow-up.41 Additional information about the methods used to estimate the TDPS and match patients is provided in supplemental Figure 2.
To assess the degree of balance between IVIg users and nonusers obtained with propensity matching, for each covariate included in the propensity score, we calculated a standardized difference (mean between-group difference divided by the pooled within-group standard deviation). Conventionally, a difference ≥0.10 standard deviations is held to be potentially important.42 We also indirectly assessed balance on measured and unmeasured characteristics by comparing the cumulative incidence of all-cause mortality and hospitalization for pneumonia in IVIg users and nonusers. If the groups were balanced on all baseline factors, we would expect IVIg users to have similar overall mortality and a lower rate of hospitalization for pneumonia and other infections compared with nonusers, based on results from randomized controlled trials.2
Cox regression was used to assess the hazards of arterial and venous TEEs in IVIg users relative to nonusers. To assess the temporal relationship between IVIg use and TEE risk, crude event rates and hazard ratio (HR) estimates from the Cox regression models were calculated for each exposure window (days 0 to 1, 2 to 15, 16 to 30, and 31 to 90 after IVIg) relative to the nonuser comparison group. To account for the matched study design, we treated each matched set as a cluster and relied on robust sandwich variance estimates.43,44 Because each IVIg user was matched to k nonusers (range, 1-3), IVIg users received a weight of 1 and nonusers a weight of 1/k in both the standardized difference calculations and the Cox regression models.45
To make our findings more clinically interpretable, we calculated a 1-year cumulative incidence difference and number needed to harm (NNH) for each of the TEE end points, representing the estimated absolute risk attributable to receiving 1 year of monthly IVIg infusions within the study population of IVIg users. These estimates were based on the Kaplan-Meier cumulative incidence of TEE observed in the unexposed patients and the model-based HR associated with the day 0 to 30 after IVIg exposure period.46
Data manipulation and statistical analyses were performed with SAS 9.4 for Windows (SAS Institute, Inc., Cary, NC).
Results
A total of 2771 patients met eligibility criteria and initiated IVIg therapy during the study period, of whom 2724 (2075 with CLL and 649 with MM) were successfully matched to 8035 nonusers. Among matched IVIg users, the median age was 75 years (range, 66-99 years), 41% were female, and the median year of treatment initiation was 2005 (range, 1992-2010). Noteworthy clinical characteristics of the IVIg users included high rates of hospitalization (64%), pneumonia (50%), chronic bronchitis (18%), receipt of antineoplastic chemotherapy (59%), and comorbid conditions such as coronary heart disease (36%) and heart failure (27%) recorded during the prior year.
Propensity matching produced user and nonuser groups that were comparable across measured demographic and clinical characteristics; for all covariates, between-group differences were <0.10 standard deviations (Table 1; comparisons across all covariates included in the propensity model can be found in supplemental Table 4). Prior to propensity matching, IVIg users were substantially more likely than nonusers to have had serious infections and a hospital stay during the last year; in general, the prevalence of other TEE risk factors was also higher among the IVIg users (supplemental Table 4). Between-group differences in the risk of mortality and hospitalization for pneumonia over the 1-year follow-up period were substantially reduced by propensity matching (supplemental Table 6); after propensity matching, all-cause mortality during follow-up was similar across IVIg users (26%) and nonusers (26%), although IVIg users were more likely to be hospitalized for pneumonia (15% vs 12%).
Over the 1-year follow-up period, the hazard of an arterial TEE among IVIg users was significantly elevated during days 0 to 1 following IVIg treatments (HR = 3.40, 95% confidence interval [CI]: 1.25, 9.25) relative to nonusers. As the number of days since a patient had received IVIg increased, the relative hazard of arterial TEE declined (HR for days 2-15 after IVIg: 1.56, 95% CI: 0.86, 2.80; HR for days 16-30 after IVIg: 0.88, 95% CI: 0.43, 1.81; Table 2). The average relative hazard associated with days 0 to 30 after IVIg was 1.38 (95% CI: 0.89, 2.14).
These analyses were repeated for the secondary venous and composite TEE end points. No venous TEE hospitalizations were observed on days 0 to 1 following IVIg treatment, leading us to combine the days 0 to 1 and days 2 to 15 exposure categories. During days 0 to 30 following treatment with IVIg, the hazard of a venous TEE among IVIg users was nonsignificantly elevated (HR = 1.27, 95% CI: 0.67, 2.41) relative to nonusers. We did not see evidence for a dose-response relationship between IVIg exposure recency and the hazard of venous TEE (Table 3). For the composite end point, the relative hazard of a TEE was nonsignificantly elevated among IVIg users during days 0 to 30 after IVIg (HR = 1.36, 95% CI: 0.95, 1.96; Table 4).
In terms of absolute risk, for patients treated monthly with IVIg over a 1-year period, the estimated increases in risk attributable to IVIg were 0.7% (95% CI: −0.2%, 2.0%), 0.3% (95% CI: −0.4%, 1.5%), and 1.0% (−0.2%, 2.7%) for the arterial, venous, and composite TEE outcomes, respectively (Table 5). These absolute risk estimates were lower than those obtained from unadjusted analyses (supplemental Table 7).
As detailed in supplemental Tables 8 and 9, we performed several post hoc sensitivity analyses to assess how our results might have differed using alternative study sample restrictions (eg, excluding patients with potential alternative indications for IVIg use), and end point definitions (eg, restricting the end point definition to outcomes for which the PPV for acute TEEs is highest). Allowing for sampling variability, the results from these sensitivity analyses were consistent with those reported above.
Within our study population, we found no evidence that the association between IVIg exposure and risk of the composite TEE outcome (expressed as a log HR) was modified by calendar year, type of cancer (CLL or MM), history of clinical cardiovascular disease, history of venous thromboembolism, hospitalization, Part D status, time since IVIg initiation, or IVIg brand. For the test of interaction by IVIg brand, we compared the TEE risks associated with (1) Flebogamma, (2) Gammagard, (3) Gamunex, and (4) a fourth group that included brand-nonspecific, Octagam, Privigen, and Carimune billing codes (combined due to sparse data). We did find evidence of interaction by age (P = .03), with the relative hazard of any TEE during days 0 to 30 following IVIg therapy increasing with age (supplemental Table 10).
Discussion
In this large, population-based cohort of patients with CLL or MM, we observed an increased risk of clinically severe arterial TEEs among new IVIg users relative to a matched group of nonusers. In particular, the hazard of an arterial TEE was increased by a factor of 3 on days 0 to 1 following IVIg infusions. When considered in terms of an increase in absolute risk averaged over a 1-year treatment period, the estimate for the primary arterial TEE end point was 0.7% (95% CI: −0.2%, 2.0%; NNH: 150, 95% CI: 50, ∞). A smaller and statistically nonsignificant attributable risk was observed for the venous TEE end point (risk difference: 0.3%, 95% CI: −0.4%, 1.5%; NNH: 340, 95% CI: 66, ∞). Our data also suggested that these risks may be higher among patients of advanced age, as is stated on the immune globulin boxed warning regarding TEE risk.7
Overall, our results are consistent with prior case reports and observational studies that have suggested an association between IVIg products and increased risk of TEE.9,10,13,14,24-32 An important contribution of our study to the existing literature is that it includes an unexposed comparison group, providing an estimate of the proportion of TEEs following IVIg that may be attributable to the IVIg itself, as opposed to a patient’s age, underlying indication for IVIg use, or other risk factors. Other study strengths include its reasonably large sample size (N = 10 759) and use of propensity matching to select an unexposed patient group comparable on a large number of measurable risk factors for thrombosis.
Limitations of this study include the potential for residual confounding and reliance on diagnoses recorded in claims data for the ascertainment of patient outcomes. Although an extensive number of risk factors could be measured from the Medicare claims data, residual confounding by factors such as cancer progression, associated hematologic abnormalities, immobility, and infection is possible. Although chart validation of outcomes was not possible, we did rely on end point definitions associated with high PPVs in prior validation studies.37-39
Last, because serious TEEs are rare events, our available sample size meant that our risk estimates are characterized by moderate sampling variability and that we had modest power to detect product-specific differences in the association between IVIg use and thrombotic risk. Data from a prior laboratory and epidemiologic assessment indicated that such differences are possible due to differences across products (and across lots of the same product) in concentrations of thrombogenic plasma constituents such as factor XIa.13 Although we did not find statistically significant differences in TEE risk across IVIg products (as identified by billing codes), we cannot rule out the possibility of clinically meaningful heterogeneity in thrombogenecity across IVIg products and lots.
We designated arterial TEEs as the primary study end point due to the acuity of onset and established high PPV of the case definition and seriousness of the event. The dose-response relationship found between recency of IVIg exposure and arterial TEE risk is consistent with the temporal pattern reported in case reports and case series.9,10,30 This finding is also consistent with the approximately 30-day half-life of exogenous IgG47,48 and hypothesized transient effect of IVIg on TEE risk.
In contrast to the primary arterial TEE endpoint, we did not find a clear temporal relationship between recency of IVIg exposure and the venous TEE secondary end point. This difference may be attributable to the potential for an extended delay between the initial formation of a venous clot, propagation of the thrombus to the point of becoming symptomatic, and hospitalization for venous TEE (our end point definition). In a previous observational study that reported on TEEs that occurred within 30 days of IVIg treatment, the median time from IVIg treatment until onset of symptomatic TEE was 1 day for arterial events vs 10 days for venous events.30 In addition, IVIg users were more likely than matched nonusers to be hospitalized for pneumonia (15% vs 12%) over the 1-year follow-up period; this suggests that the excess venous TEE risk observed among the IVIg users may be partially attributable to confounding by immobility, a strong risk factor for venous TEEs.
Clinicians and patients must weigh the possible risks associated with IVIg use, including thromboembolic adverse events, against its therapeutic benefits. In patients with humoral immunodeficiency secondary to CLL or MM, IVIg has been shown in randomized controlled trials to reduce the risk of major infection (pooled relative risk = 0.45. 95% CI: 0.27, 0.75; number needed to treat for 1 year to prevent a major infection = 5, 95% CI: 3, 13), but not overall mortality.2 Within our study population, our best estimates for the NNH are 150 (95% CI: 50, ∞) and 340 (95% CI: 66, ∞) for the clinically serious arterial and venous TEE end points, respectively. If additional evidence supports the hypothesis that these associations are genuine causal effects, the net clinical benefit of IVIg for a patient with secondary immunodeficiency may depend on the patient’s baseline risks of major infection and TEE, as well as patient preferences and quality-of-life considerations.
In terms of the generalizability of our results to other patient populations, 2 factors should be highlighted. First, patients in our study cohort primarily received IVIg for acquired humoral immunodeficiency. A standard dose for this indication is 0.4 g/kg body weight, whereas patients with certain autoimmune and inflammatory conditions may receive much higher doses (1.0-2.0 g/kg body weight).1,49 Second, patients in our study cohort were older adults with a high burden of risk factors for arterial and venous TEEs. For these reasons, our risk estimates may not be generalizable to patients receiving higher doses of IVIg for other indications or patients with a substantially different baseline risk of TEEs.
Given the heterogeneity of indications for IVIg use, including a variety of immune deficiencies and autoimmune or inflammatory conditions,49 an area for future research is to quantify the absolute risk of TEEs attributable to IVIg across different patient populations. The Mini-Sentinel safety assessment of IVIg-associated TEE risk is a large ongoing study based on chart-validated administrative data that seeks to address these questions.50 The Mini-Sentinel study, in which both IVIg treatment and TEE end points will be chart confirmed, will assess TEE risk in new IVIg users of all ages, including a large proportion of patients receiving high-dose IVIg for autoimmune and inflammatory indications. Results from this study will allow patients and clinicians to better assess the likely risk-benefit ratio for IVIg therapy for a variety of indications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank collaborators on a related protocol-based IVIg safety study, Thromboembolic Events after Immunoglobulin Administration, conducted under Task Order 6 (contract HHSF22301006T) of the Mini-Sentinel project (contract HHSF223200910006I), which was funded by the Food and Drug Administration through the Department of Health and Human Services. Work on that project provided many insights that helped to inform the exposure, end point, and covariate definitions used in this study.
This work was made possible by the University of Iowa Holden Comprehensive Cancer Center Population Research Core, which is supported in part by National Institutes of Health National Cancer Institute grant P30 CA086862.
This study used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services (CMS); Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; IMS, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
This research will constitute part of Eric Ammann’s dissertation as a Department of Epidemiology PhD candidate at the University of Iowa. As a graduate student, he has received tuition and salary support through a Presidential Graduate Research Fellowship from the University of Iowa Graduate College.
Authorship
Contribution: E.M.A. contributed to the research design, analysis, and interpretation of the data and manuscript writing and revision; M.P.J. contributed to the research design and analysis and interpretation of the data; B.K.L. contributed to the analysis and interpretation of the data and manuscript revision; R.M.C. contributed to the definitions of exposure, end points, and covariates and to the analysis and interpretation of the data; S.K.W. contributed to the definitions of exposure, end points, and covariates and to the analysis and interpretation of the data; J.C.T. contributed to the analysis and interpretation of the data; B.D.M. contributed to the analysis and interpretation of the data; B.H.F. contributed to the analysis and interpretation of the data; and E.A.C. contributed to the research design, analysis, and interpretation of the data and manuscript writing and revision.
Conflict-of-interest disclosure: E.M.A. has received salary support in 2013 to 2015 for research on a related IVIg safety study under Task Order 6 (contract HHSF22301006T) of the Mini-Sentinel project (contract HHSF223200910006I), which was funded by the Food and Drug Administration through the Department of Health and Human Services. R.M.C. has received salary support in 2013 to 2015 for a related Mini-Sentinel IVIg safety study, as described above. S.K.W. was co-lead of a related Mini-Sentinel IVIg safety study, as described above. J.C.T. has received salary support in 2013 to 2015 for a related Mini-Sentinel IVIg safety study, as described above. B.H.F. has received salary support in 2013 to 2015 for a related Mini-Sentinel IVIg safety study, as described above. E.A.C. has received salary support in 2013-15 for a related Mini-Sentinel IVIg safety study, as described above. All other authors declare no competing financial interests.
Correspondence: Eric Ammann, 145 N Riverside Dr, Department of Epidemiology–400 CPHB, Iowa City, IA 52242; e-mail: eric-ammann@uiowa.edu.