Key Points
Mice lacking both WASP and N-WASP in B lymphocytes have impaired response to T-cell-dependent antigens and defective B-cell activation.
Deletion of N-WASP in B cells attenuates autoimmunity in WASP-deficient mice.
Abstract
Mutations of the Wiskott-Aldrich syndrome gene (WAS) are responsible for Wiskott-Aldrich syndrome (WAS), a disease characterized by thrombocytopenia, eczema, immunodeficiency, and autoimmunity. Mice with conditional deficiency of Was in B lymphocytes (B/WcKO) have revealed a critical role for WAS protein (WASP) expression in B lymphocytes in the maintenance of immune homeostasis. Neural WASP (N-WASP) is a broadly expressed homolog of WASP, and regulates B-cell signaling by modulating B-cell receptor (BCR) clustering and internalization. We have generated a double conditional mouse lacking both WASP and N-WASP selectively in B lymphocytes (B/DcKO). Compared with B/WcKO mice, B/DcKO mice showed defective B-lymphocyte proliferation and impaired antibody responses to T-cell-dependent antigens, associated with decreased autoantibody production and lack of autoimmune kidney disease. These results demonstrate that N-WASP expression in B lymphocytes is required for the development of autoimmunity of WAS and may represent a novel therapeutic target in WAS.
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked disease characterized by eczema, thrombocytopenia, immunodeficiency, and autoimmunity.1,2 By generating a mouse lacking expression of the WAS protein (WASP) selectively in B lymphocytes (B/WcKO), we and others have revealed a nonredundant B-cell-intrinsic role of WASP in immune homeostasis and prevention of autoimmunity, as well as in marginal zone (MZ) development and regulation of the germinal center (GC) reaction.3-5 Neural WASP (N-WASP, encoded by the Wasl gene) is another member of the WASP family of proteins; it is ubiquitously expressed and shares 50% homology with WASP.6 Similar to WASP, N-WASP undergoes a conformational change upon activation that enables initiation of actin polymerization,7,8 thereby linking cellular activation to cytoskeletal modifications.9 Selective deletion of N-WASP in B lymphocytes of Was knockout (WKO) mice resulted in the aggravation of B-cell abnormalities, including a strong decrease of intracellular calcium flux and Bruton’s tyrosine kinase (Btk) and Src homology 2-containing inositol 5′ phosphatase phosphorylation upon B-cell receptor (BCR) stimulation,10 further worsening of MZ B-cell depletion,11 and defective somatic hypermutation.12 However, lack of WASP expression in multiple hematopoietic cells may have indirectly contributed to B-cell abnormalities in these models.
To investigate the B-cell intrinsic role played by WASP and N-WASP in immune homeostasis and regulation more specifically, we have developed a double conditional mouse model (B/DcKO) in which deletion of both Was and Wasl floxed alleles in B lymphocytes is driven by the Cre recombinase expressed under the B-cell-specific promoter mb1. Here we show that deletion of N-WASP in B cells impairs B-cell activation and T-cell-dependent antibody responses and reduces manifestations of immune dysregulation seen in B/WcKO mice.
Methods
Detailed methods are presented in supplemental Data (available on the Blood Web site). B/DcKO mice were generated by breeding B/WcKO3 with Waslfl/fl 13 mice. Lymphocyte subsets were analyzed by fluorescence-activated cell sorting (FACS) and immunofluorescence staining of spleen sections. FACS-sorted spleen follicular (Fo) and MZ B cells were analyzed for proliferation by assessing carboxyfluorescein succinimidyl ester dilution at day 4 after stimulation with anti-immunoglobulin M (anti-IgM) and CpG 1826. Intraperitoneal immunization with 2,4,6-trinitrophenyl hapten-Keyhole limpet hemocyanin (TNP-KLH) was performed as described.14 Ig serum levels were analyzed by enzyme-linked immunosorbent assay.14 Levels of serum autoantibodies were assessed by enzyme-linked immunosorbent assay or by using a protein array (University of Texas Southwestern Medical Center).3,15 Pathological scoring of periodic acid–Schiff-stained kidney sections from 7- to 20-month-old mice was assessed blindly by a trained nephrologist as previously described.3
Results and discussion
FACS analysis of the B-cell compartment yielded similar results in B/DcKO and B/WcKO mice. In particular, B-lymphocyte progenitors were normally represented in the bone marrow of B/DcKO mice (supplemental Figure 1A-B); however, the proportion of B220hiIgM+ bone marrow mature recirculating B cells was markedly reduced (Figure 1A). Furthermore, B/DcKO mice had a normal frequency and absolute count of transitional and mature Fo B cells in the spleen (supplemental Figure 1C-D) but a marked reduction of MZ B cells (Figure 1B). Analysis of serum Ig levels showed that B/DcKO mice lacked the increase of IgM and IgE serum levels observed in WKO and B/WcKO mice3 and had lower IgG levels (supplemental Figure 2). The distribution and count of CD4+ and CD8+ splenic T cells were unaffected in B/DcKO mice (data not shown).
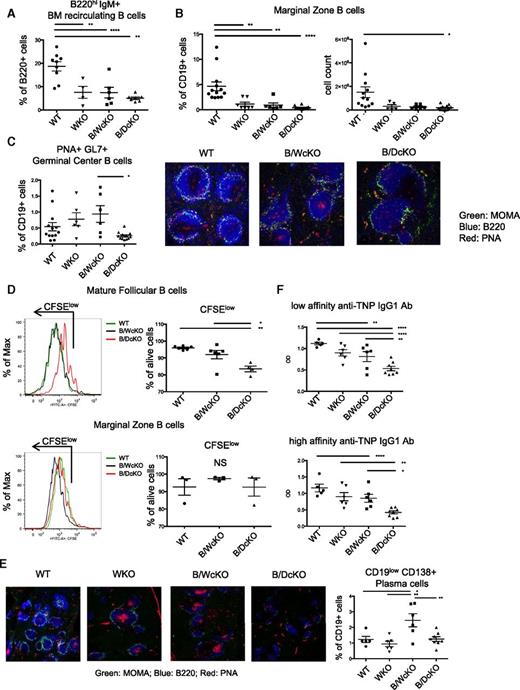
N-WASP deletion impairs GC formation and causes defective B-cell response in vitro and in vivo. Flow cytometry analysis showing severe reduction of the proportion of (A) bone marrow (BM) recirculating B220hi lymphocytes and (B) splenic MZ B cells in all indicated knockout models as compared with wild-type (WT) mice. (C) Left panel: Reduced proportion of PNA+GL7+ GC B cells in naïve B/DcKO compared with naïve B/WcKO mice. Right panels: Immunofluorescence staining of optimal cutting temperature frozen spleen sections with anti-B220 (blue), anti-monocyte plus macrophage (anti-MOMA; green) monoclonal antibodies, and peanut agglutinin (PNA; red) demonstrating the presence of PNA+ GC B cells in naïve B/WcKO mice, but not in unimmunized WT and B/DcKO mice. Marked reduction of B220+ lymphocytes surrounding MOMA+ macrophages in B/WcKO and in B/DcKO mice is indicative of MZ defect. Shown are representative images from 3 different mice per group for WT and B/DcKO (original magnification ×20). (D) Impaired proliferation (as indicated by dilution of carboxyfluorescein succinimidyl ester (CFSE) and viability of sorted spleen Fo (upper panels) but not MZ (lower panels) B cells from B/DcKO mice upon stimulation with anti-IgM and CpG. (E) Immunofluorescence staining of spleen sections reveals a reduction of PNA+ GC formation in B/DcKO mice at day 7 after boosting immunization with TNP-KLH. Shown are representative images from 3 different mice per group for WT and B/DcKO (original magnification ×10). (F) Defective IgG1-specific anti-TNP responses in B/DcKO mice upon immunization with TNP-KLH. Data from individual mice are shown in representative dot plots. Statistical analysis was performed by one-way analysis of variance with Bonferroni post hoc analysis (FACS analysis of lymphocyte populations) and two-way analysis of variance with Bonferroni post hoc analysis (enzyme-linked immunosorbent assay detection of anti-TNP antibodies). *P < .05, **P < .01, ****P < .0001. OD, optical density.
N-WASP deletion impairs GC formation and causes defective B-cell response in vitro and in vivo. Flow cytometry analysis showing severe reduction of the proportion of (A) bone marrow (BM) recirculating B220hi lymphocytes and (B) splenic MZ B cells in all indicated knockout models as compared with wild-type (WT) mice. (C) Left panel: Reduced proportion of PNA+GL7+ GC B cells in naïve B/DcKO compared with naïve B/WcKO mice. Right panels: Immunofluorescence staining of optimal cutting temperature frozen spleen sections with anti-B220 (blue), anti-monocyte plus macrophage (anti-MOMA; green) monoclonal antibodies, and peanut agglutinin (PNA; red) demonstrating the presence of PNA+ GC B cells in naïve B/WcKO mice, but not in unimmunized WT and B/DcKO mice. Marked reduction of B220+ lymphocytes surrounding MOMA+ macrophages in B/WcKO and in B/DcKO mice is indicative of MZ defect. Shown are representative images from 3 different mice per group for WT and B/DcKO (original magnification ×20). (D) Impaired proliferation (as indicated by dilution of carboxyfluorescein succinimidyl ester (CFSE) and viability of sorted spleen Fo (upper panels) but not MZ (lower panels) B cells from B/DcKO mice upon stimulation with anti-IgM and CpG. (E) Immunofluorescence staining of spleen sections reveals a reduction of PNA+ GC formation in B/DcKO mice at day 7 after boosting immunization with TNP-KLH. Shown are representative images from 3 different mice per group for WT and B/DcKO (original magnification ×10). (F) Defective IgG1-specific anti-TNP responses in B/DcKO mice upon immunization with TNP-KLH. Data from individual mice are shown in representative dot plots. Statistical analysis was performed by one-way analysis of variance with Bonferroni post hoc analysis (FACS analysis of lymphocyte populations) and two-way analysis of variance with Bonferroni post hoc analysis (enzyme-linked immunosorbent assay detection of anti-TNP antibodies). *P < .05, **P < .01, ****P < .0001. OD, optical density.
We have previously shown that spontaneous GC formation is a prominent feature of immune dysregulation in B/WcKO mice.3 By contrast, B/DcKO mice did not present spontaneous GC formation, as shown by the low proportion of PNA+GL7+ GC B cells and lack of peanut agglutinin staining in the spleen follicles of naïve mice (Figure 1C). These results suggest that concurrent deletion of N-WASP in the B-cell lineage of B/WcKO mice restrains spontaneous GC formation.
To test the hypothesis that the combined N-WASP and WASP deletion may affect B-cell activation, we stimulated sorted spleen Fo and MZ B cells from B/DcKO, B/WcKO, and wild-type mice with anti-IgM antibody and CpG. Upon in vitro stimulation, proliferation and viability of B/DcKO Fo B lymphocytes, but not MZ B cells, were markedly impaired (Figure 1D). These data are consistent with data recently reported by others.10 To determine whether these functional abnormalities of B/DcKO Fo B cells may have important implications in vivo, we immunized mice with the T-cell-dependent antigen TNP-KLH. Upon immunization, robust GC formation (as indicated by peanut agglutinin staining) and an increased proportion of CD19lowCD138+ plasma cells were observed in the spleens of B/WcKO but not B/DcKO mice (Figure 1E). Furthermore, both low- and high-affinity IgG1 anti-TNP antibody responses were reduced in B/DcKO compared with B/WcKO mice (Figure 1F). Altogether, these data indicate that activation of Fo B cells and in vivo response to T-cell-dependent antigens are impaired in B/DcKO mice.
Autoimmunity is a prominent feature in B/WcKO mice, with increased production of IgM and IgG autoantibodies (Figure 2A-B and supplemental Figure 3A).6 By contrast, B/DcKO mice lacked IgG autoantibodies to double-stranded DNA and single-stranded DNA (Figure 2A) and to a broad range of self-antigens, as tested by a protein array (Figure 2B). However, they showed increased levels of IgM autoantibodies, which were also observed in WKO and B/WcKO mice (supplemental Figure 3A). Moreover, unimmunized B/DcKO mutant mice had higher levels of IgM anti-TNP polyreactive antibodies (supplemental Figure 3B), as previously shown for B/WcKO mice.3 Finally, whereas older B/WcKO and especially WKO mice developed kidney immunopathology, as previously reported,6 none of the B/DcKO mice studied (up to age 14 months) showed signs of kidney disease (Figure 2C-D). All three mutant strains, but not wild-type mice, had increased glomerular deposits of IgG. No differences in the amount of IgM, IgG, or C3 deposits were observed among WKO, B/WcKO, and B/DcKO mice (supplemental Figure 4). Overall, in comparison with B/WcKO mice, B/DcKO mice show reduced levels of IgG autoantibodies and lack of tissue immunopathology in spite of the presence of IgM autoantibodies.
N-WASP deletion protects mice from autoimmune manifestations. (A) Anti-single-stranded DNA (ssDNA) and anti-double-stranded DNA (dsDNA) IgG antibodies as measured by enzyme-linked immunosorbent assay and (B) detection of IgG autoantibodies by protein array in the serum of indicated mice. Results are shown for autoantibodies with significantly decreased reactivity (as assessed by significance analysis of microarray with false discovery rate <1) in B/DcKO mice compared with B/WcKO mice. Shown are heat maps of net fluorescence intensity ratios between each mouse and the average plus 2 standard deviations for the net fluorescence intensity ratios for WT mice for each antigen. Range is from 0 (blue) to 1 (black) to 5 (yellow). Sera from systemic lupus erythematosus–prone MRL-lpr/lpr mice were used as a positive control. (C) Periodic acid–Schiff staining of formalin-fixed paraffin-embedded kidney sections reveals severe hypercellularity and capillary wall thickening in WKO and B/WcKO mice, but not in B/DcKO mice age 7 to 14 months. Representative tissue pathology of 8 to 15 mice per group is shown. (D) Blinded pathological glomerular scoring (ranging from 0 [no glomerular pathology] to 3 [severe glomerular pathology]) performed by a nephropathologist, confirming kidney damage in WKO and B/WcKO, but not in B/DcKO mice. Statistical significance was assessed by Mann-Whitney test (enzyme-linked immunosorbent assay detection of autoantibodies and pathological score) and significance analysis of microarray (protein array). *P < .05, **P < .01, ***P < .001).
N-WASP deletion protects mice from autoimmune manifestations. (A) Anti-single-stranded DNA (ssDNA) and anti-double-stranded DNA (dsDNA) IgG antibodies as measured by enzyme-linked immunosorbent assay and (B) detection of IgG autoantibodies by protein array in the serum of indicated mice. Results are shown for autoantibodies with significantly decreased reactivity (as assessed by significance analysis of microarray with false discovery rate <1) in B/DcKO mice compared with B/WcKO mice. Shown are heat maps of net fluorescence intensity ratios between each mouse and the average plus 2 standard deviations for the net fluorescence intensity ratios for WT mice for each antigen. Range is from 0 (blue) to 1 (black) to 5 (yellow). Sera from systemic lupus erythematosus–prone MRL-lpr/lpr mice were used as a positive control. (C) Periodic acid–Schiff staining of formalin-fixed paraffin-embedded kidney sections reveals severe hypercellularity and capillary wall thickening in WKO and B/WcKO mice, but not in B/DcKO mice age 7 to 14 months. Representative tissue pathology of 8 to 15 mice per group is shown. (D) Blinded pathological glomerular scoring (ranging from 0 [no glomerular pathology] to 3 [severe glomerular pathology]) performed by a nephropathologist, confirming kidney damage in WKO and B/WcKO, but not in B/DcKO mice. Statistical significance was assessed by Mann-Whitney test (enzyme-linked immunosorbent assay detection of autoantibodies and pathological score) and significance analysis of microarray (protein array). *P < .05, **P < .01, ***P < .001).
Recent data have suggested a direct role of WASP in shaping the immune repertoire through negative selection of autoreactive progenitors16 and skewing of the BCR repertoire.17 The strength of BCR signaling controls central mechanisms of B-cell tolerance, including deletion, receptor editing, and anergy.18-20 We have shown that simultaneous deletion of WASP and N-WASP affects proliferation of Fo B cells but not MZ B cells in response to BCR and Toll-like receptor 9 (TLR9) signaling. Our results support a model in which deletion of both WASP and N-WASP causes a defect in the BCR signaling responsible for the accumulation of IgM autoantibody-secreting plasma cells, whereas GC responses and generation of class-switched autoreactive B cells are impaired.10,12 Defective migration of B cells devoid of both WASP and N-WASP to the GC12 and impaired class switching in response to CD40 or TLR signaling may both contribute to this defect. Finally, although both WKO and B/WcKO mice had similar levels of IgM autoantibodies and higher levels of IgG autoantibodies were observed in B/WcKO mice, renal damage was more severe in WKO mice. No tissue immunopathology was observed in B/DcKO mice in spite of IgM autoantibodies. These apparent discrepancies may be reconciled with the observation that other blood lineages, in particular regulatory T cells and plasmacytoid dendritic cells, play important, WASP-dependent roles in immune homeostasis,21-24 independently of autoantibody production.25 In conclusion, our data broaden the understanding of the molecular mechanisms underlying immune dysregulation in WAS and suggest that N-WASP may be an attractive novel target for pharmacological control of autoimmunity in patients with this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant 5P01HL059561 from the National Heart, Lung, and Blood Institute, National Institutes of Health (L.D.N.) and grant CHUV-UNIL CGRB 29583 from the University Hospital of Lausanne and the Suisse National Science Foundation (F.C.). A.J.T. is supported by the Wellcome Trust.
Authorship
Contribution: S.V. performed and analyzed most experiments and wrote the manuscript; E.S., K.A., C.I.M.D., M.R., K.C., E.C., D.R., and D.M. performed experiments; M.M. performed experiments and contributed to the writing of the paper; G.C.T. and L.S.W. supervised some experiments; S.S. and A.J.T. contributed vital experimental tools; F.C. supervised some experiments and contributed to the writing of the paper; and L.D.N. designed, analyzed, and supervised all experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi D. Notarangelo, Division of Immunology, Boston Children’s Hospital, Karp Research Building, Room 10217, 1 Blackfan Circle, Boston, MA 02115; e-mail: luigi.notarangelo@childrens.harvard.edu.

![Figure 2. N-WASP deletion protects mice from autoimmune manifestations. (A) Anti-single-stranded DNA (ssDNA) and anti-double-stranded DNA (dsDNA) IgG antibodies as measured by enzyme-linked immunosorbent assay and (B) detection of IgG autoantibodies by protein array in the serum of indicated mice. Results are shown for autoantibodies with significantly decreased reactivity (as assessed by significance analysis of microarray with false discovery rate <1) in B/DcKO mice compared with B/WcKO mice. Shown are heat maps of net fluorescence intensity ratios between each mouse and the average plus 2 standard deviations for the net fluorescence intensity ratios for WT mice for each antigen. Range is from 0 (blue) to 1 (black) to 5 (yellow). Sera from systemic lupus erythematosus–prone MRL-lpr/lpr mice were used as a positive control. (C) Periodic acid–Schiff staining of formalin-fixed paraffin-embedded kidney sections reveals severe hypercellularity and capillary wall thickening in WKO and B/WcKO mice, but not in B/DcKO mice age 7 to 14 months. Representative tissue pathology of 8 to 15 mice per group is shown. (D) Blinded pathological glomerular scoring (ranging from 0 [no glomerular pathology] to 3 [severe glomerular pathology]) performed by a nephropathologist, confirming kidney damage in WKO and B/WcKO, but not in B/DcKO mice. Statistical significance was assessed by Mann-Whitney test (enzyme-linked immunosorbent assay detection of autoantibodies and pathological score) and significance analysis of microarray (protein array). *P < .05, **P < .01, ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/2/10.1182_blood-2015-05-643817/4/m_216f2.jpeg?Expires=1765080554&Signature=zyELjGcamzblWish0qpKmlnNtmFAQo~fIJl~xkaB-wLdByFF6RMDkBoCCbXaGLKeeb0asV0SPzuI7yEsdySu-DF48K~sVMDgmfqlxYaSETJaEUeIa5lKH7jaSx5gwiRdo5hiEIJW1jJz355Ob3LqX0tGZwbkyti20UgUf087ho1~~jz2kzVIlFrsHpwncGfTLFD~GpdSu9oVuWhoWUlVA6WDCAG5lQP7ePtOPR8~ka8KuCrdptlmxEL15dx6OQO8U4H~q0NgQx1Bn16Ky5Bcaehxji3ifTJfX5LViHqP-16RvTlc5~3bfWKDEuZQMoH~CKeC-MgbCqZmEidjmZfi2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
