Key Points
In mice, loss of GNA13 in GC B cells protects against cell death and may promote genetic instability via ongoing somatic hypermutation.
Gna13 loss, in combination with MYC overexpression, promotes lymphoma in mice.
Abstract
GNA13 is the most frequently mutated gene in germinal center (GC)-derived B-cell lymphomas, including nearly a quarter of Burkitt lymphoma and GC-derived diffuse large B-cell lymphoma. These mutations occur in a pattern consistent with loss of function. We have modeled the GNA13-deficient state exclusively in GC B cells by crossing the Gna13 conditional knockout mouse strain with the GC-specific AID-Cre transgenic strain. AID-Cre+ GNA13-deficient mice demonstrate disordered GC architecture and dark zone/light zone distribution in vivo, and demonstrate altered migration behavior, decreased levels of filamentous actin, and attenuated RhoA activity in vitro. We also found that GNA13-deficient mice have increased numbers of GC B cells that display impaired caspase-mediated cell death and increased frequency of somatic hypermutation in the immunoglobulin VH locus. Lastly, GNA13 deficiency, combined with conditional MYC transgene expression in mouse GC B cells, promotes lymphomagenesis. Thus, GNA13 loss is associated with GC B-cell persistence, in which impaired apoptosis and ongoing somatic hypermutation may lead to an increased risk of lymphoma development.
Introduction
Non-Hodgkin lymphomas are classified on the basis of the immune cells from which they arise, with the majority deriving from germinal center (GC) B cells, including Burkitt lymphoma and half of diffuse large B-cell lymphoma (DLBCL), termed GC B-cell subtype DLBCL.1
GCs are transient structures that form in secondary lymphoid organs upon antigenic stimulation. After encountering antigen, B cells home to the GC dark zone (DZ) to undergo rapid proliferation, somatic hypermutation (SHM) of the immunoglobulin genes, and class switch recombination. B cells then migrate to the GC light zone (LZ), where they undergo affinity selection. Repeated rounds of chemokine-directed DZ/LZ cycling create GC B cells with specific, robust antibody responses.2
The process of SHM, in which antibody diversity is enhanced by the introduction of mutations into the variable region of the immunoglobulin genes, represents a distinct genetic vulnerability of GC B cells, because off-target somatic mutations in other genes can also occur.3 The unique biology of the GC B-cell differentiation state is reflected in the distinct mutational spectrum of tumors arising from this cell type.4,5 GNA13 mutations are among the most common genetic alterations in GC-derived B-cell lymphomas, with mutations identified in nearly a quarter of Burkitt lymphoma5 and GC B-cell subtype DLBCL.4 In contrast to its high mutational frequency in GC-derived B-cell lymphomas, genetic events in GNA13 are largely absent from all other hematologic malignancies, including non-GC B-cell lymphomas.4,6-9 The manner in which GNA13 mutations promote lymphoma is not fully understood.
Recent work by Muppidi et al used mouse chimeric models and B lineage–specific Mb1-Cre+Gna13 conditional knockout mice to demonstrate that Gna13 deficiency is associated with GC B-cell population expansion and dissemination beyond the GC, increased GC B-cell survival associated with elevated levels of phosphorylated protein kinase B (pAKT), and susceptibility to lymphoma in aged mice.10 In the present study, we use a GC-specific Gna13 knockout mouse model to understand how altered B-cell migration and impaired cell death might contribute to oncogenesis within the GC niche.
Methods
B-cell isolation and RhoA activation assay
B cells were purified from freshly isolated splenocytes using a MACS B Cell Isolation Kit (Miltenyi Biotec). RhoA pull-down assay was performed using the RhoA Pull-down Activation Assay Biochem Kit (Cytoskeleton).
Immunofluorescence
Immunized AID-Cre+Gna13 mice were euthanized and perfused with 4% paraformaldehyde in phosphate-buffered saline. Cells were stained overnight with GL7–fluorescein isothiocyanate (supplemental Appendix, available on the Blood Web site) diluted 1:100 in blocking buffer. Images were obtained using a Zeiss 710 laser scanning confocal microscope using a ×40 oil (1.3NA) objective lens.
Flow cytometry of murine cells
Mouse spleen, Peyer patches, and bone marrow were harvested in RPMI 1640 medium containing 10% (v/v) fetal bovine serum. For detailed staining protocols and antibodies, see supplemental Figure 6 and supplemental Appendix.
Transwell migration assays
Freshly isolated mouse splenocytes were used. Medium alone or containing 100 ng/mL CXCL12 (Peprotech) was placed in the lower well of 5-μm transwell chambers (Corning). Cells were placed in the upper chamber and allowed to migrate for 3 hours at 37°C. Input and migrated populations were stained to identify GC B cells and quantitated by flow cytometry.
SHM analysis
GC B and follicular (FO) B cells were isolated from AID-Cre+Gna13 mice injected with hapten and from Mb1-Cre+Gna13 mice injected with sheep red blood cells. Polymerase chain reaction (PCR) amplification of the JH4 intronic region was performed as described previously.11 The PCR product was sequenced using 300-bp paired-end reads on the Illumina platform. Reads were aligned using Bowtie2. Nonreference bases with a base quality >30 were counted at each position and converted to mutational frequencies.
Results
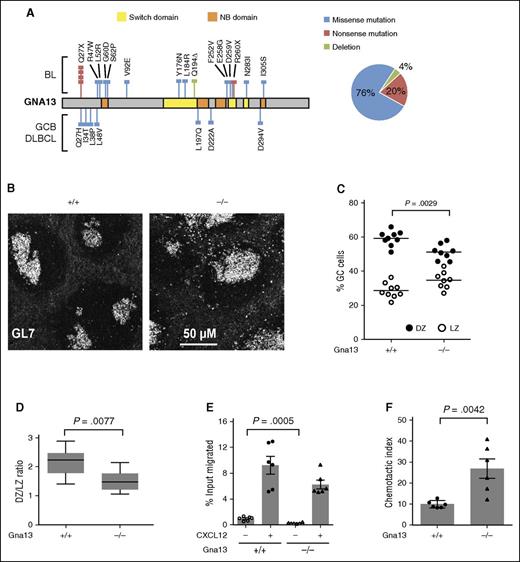
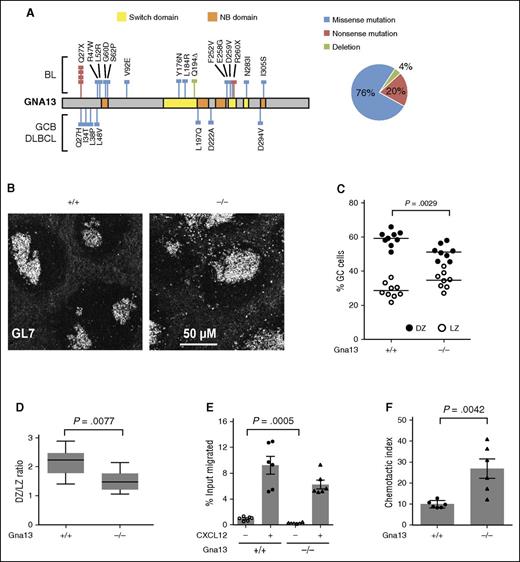
Genetic alterations in GNA13 identified in GC-derived B-cell lymphomas, including copy number loss, frameshift, nonsense, and other damaging mutations in conserved functional domains are predicted to result in a loss of protein function7 (Figure 1A; supplemental Figure 1). We therefore investigated the functional consequences of GNA13 loss in GC B cells using AID-Cre+Gna13 conditional knockout mice, in which Gna13 is disrupted at the GC B-cell stage of B-cell differentiation. In addition, the Mb1-Cre conditional model was used for validation and for assays requiring B-cell isolation (supplemental Figures 2 and 3). We first evaluated GC architecture in spleens isolated from AID-Cre+Gna13 mice using immunofluorescence. Wild-type GC B cells demonstrated tight restriction to the GC, whereas Gna13-null GC B cells were less confined, with a number of GL7+ cells appearing outside the GC (Figure 1B). The overall GC number was not altered by GNA13 deficiency (supplemental Figure 4A); however, there was an increase in the GC area in Gna13-null spleens (supplemental Figure 4B, P = .0402), which may reflect a reduction of GC B-cell confinement to the GC boundary and/or an overall increase GC B cells. These findings are consistent with the previous description of GC architecture in the Mb-1 Cre model.10
GNA13 loss results in abnormal GC architecture and altered chemokine-directed migration. (A) GNA13 mutations identified by exome sequencing in GC B-cell subtype (GCB) DLBCL and Burkitt lymphoma (BL) are depicted along the length of the gene, shown in gray. Colored lines indicate the type of mutation (blue corresponding to missense, red for nonsense, and green for deletion) and the colored boxes represent important functional domains. The switch domain, shown in yellow, is important for initiating conformational changes in GNA13 that affect its guanosine triphosphate binding affinity. The nucleotide binding (NB) domain, shown in orange, forms the guanosine triphosphate binding site. The pie chart (right) shows the percentage of GNA13 mutations that are missense, nonsense, or deletion. (B) Immunofluorescent staining of GC B cells (GL7+, white) in sections of spleen from 3-month-old AID-Cre+Gna13 cohort mice. Images are representative of 3 animals per genotype. (C) Dot plot showing the percentage of CXCR4+CD83− GC cells (DZ) compared with CXCR4−CD83+ (LZ) cells in GC B cells from Peyer patches of AID-Cre+Gna13 mice. Dots represent individual animals, with DZ cells indicated in black and LZ cells indicated in white. (D) Box and whisker plot of the average DZ:LZ ratio across genotypes. Data are the summation of 3 experiments. (E) Transwell migration of splenic GC cells isolated from 2-month-old AID-Cre+Gna13 mice with and without CXCL12 stimulation. (F) Same as panel E, depicted as chemotactic index, the number of cells that migrated over 3 hours toward 100 ng/mL CXCL12 divided by the number of cells that migrated in the absence of chemokine. Data are the representative of 4 experiments.
GNA13 loss results in abnormal GC architecture and altered chemokine-directed migration. (A) GNA13 mutations identified by exome sequencing in GC B-cell subtype (GCB) DLBCL and Burkitt lymphoma (BL) are depicted along the length of the gene, shown in gray. Colored lines indicate the type of mutation (blue corresponding to missense, red for nonsense, and green for deletion) and the colored boxes represent important functional domains. The switch domain, shown in yellow, is important for initiating conformational changes in GNA13 that affect its guanosine triphosphate binding affinity. The nucleotide binding (NB) domain, shown in orange, forms the guanosine triphosphate binding site. The pie chart (right) shows the percentage of GNA13 mutations that are missense, nonsense, or deletion. (B) Immunofluorescent staining of GC B cells (GL7+, white) in sections of spleen from 3-month-old AID-Cre+Gna13 cohort mice. Images are representative of 3 animals per genotype. (C) Dot plot showing the percentage of CXCR4+CD83− GC cells (DZ) compared with CXCR4−CD83+ (LZ) cells in GC B cells from Peyer patches of AID-Cre+Gna13 mice. Dots represent individual animals, with DZ cells indicated in black and LZ cells indicated in white. (D) Box and whisker plot of the average DZ:LZ ratio across genotypes. Data are the summation of 3 experiments. (E) Transwell migration of splenic GC cells isolated from 2-month-old AID-Cre+Gna13 mice with and without CXCL12 stimulation. (F) Same as panel E, depicted as chemotactic index, the number of cells that migrated over 3 hours toward 100 ng/mL CXCL12 divided by the number of cells that migrated in the absence of chemokine. Data are the representative of 4 experiments.
The specific effect of Gna13 deletion on GC LZ and DZ populations has not been previously reported. Under normal conditions, DZ and LZ subpopulations have a characteristic ratio of 2:1 in both humans and mice.12 We found that AID-Cre+Gna13-deficient animals had a higher fraction of GC B cells expressing LZ markers compared with wild-type (Figure 1C, P = .0029), resulting in an altered DZ:LZ ratio of 1.5:1 (Figure 1D, P = .0077). The disruption of DZ:LZ proportionality observed in the GNA13-deficient state likely reflects a derangement of the highly regulated patterns of cell migration intrinsic to DZ/LZ biology.
To determine whether the diminished DZ:LZ ratio might be due to altered LZ-directed migration, we measured GC B-cell chemotaxis in vitro in the presence of CXCL12. Compared with wild-type, GNA13-deficient GC B cells from AID-Cre+ mice demonstrated significantly altered migration patterns in transwell migration assays. We found that GNA13-deficient GC B cells migrate less than wild-type cells under basal conditions (P = .0005), but not under chemokine-stimulated conditions (Figure 1E). The reduced basal migration observed the GNA13-deficient GC B cells leads to an increased chemotactic index, which reflects the relative change in chemotaxis between basal and stimulated states (Figure 1F, P = .0042). The previous study using the Mb1-Cre+Gna13 mouse reported no difference in CXCL12-mediated chemotaxis, likely because the data were analyzed by percentage input rather than chemotactic index. Reduced basal migration, however, is also demonstrated in those data. The altered migration response observed here, in conjunction with the reduced effect of the inhibitory chemokine sphingosine-1-phosphate noted previously,10 may contribute to an abnormal GC zonal ratio.
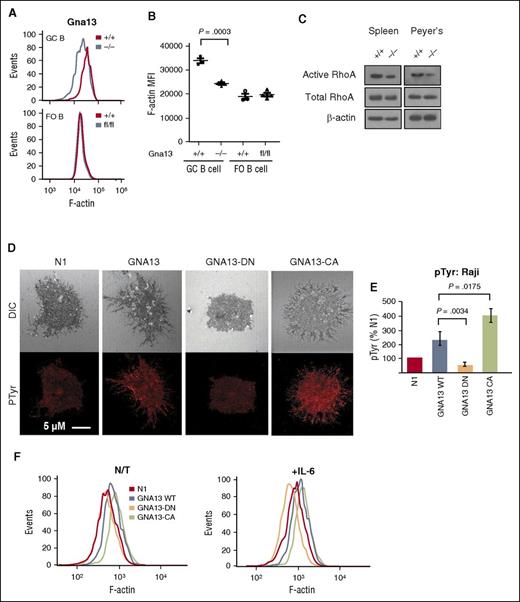
We next tested whether cellular adhesion, a function required for affinity maturation in the GC LZ,13 might be altered in the setting of GNA13 deficiency. Filamentous actin (F-actin) is known to accumulate at focal adhesion sites such as the immunologic synapse in B lymphocytes.14 Therefore, we measured F-actin expression in GC B cells from AID-Cre+Gna13 mice. GNA13-deficient GC cells demonstrated reduced F-actin compared with wild-type GC B cells and FO B-cell controls (Figure 2A-B, spleen, P = .0003; supplemental Figure 4C, Peyer patches, P = .0032). This reduction in F-actin was reproducible in Mb1-Cre+Gna13–deficient animals (supplemental Figure 4D, spleen, P = .0036; supplemental Figure 4E, Peyer patches, P = .0082). Although a variety of processes can affect the cellular content of F-actin, including nucleation and branching factors, myosin phosphorylation levels, and the activity of actin filament disassembly proteins, GNA13 is believed to regulate actin polymerization in part by altering the activity of RhoA GTPase leading to inhibition of cofilin-mediated actin disassembly.15 Reduced RhoA activity has been shown to decrease B-cell adhesion in vitro,16 but has not been studied in the context of GNA13-deficient GC B cells. We thus compared levels of total and guanosine triphosphate–bound (active) RhoA in B-cell lysates isolated from spleens and Peyer patches from Mb1-Cre+Gna13 mice (Figure 2C). We found that B cells from Gna13-null mice had at least twofold less guanosine triphosphate–bound RhoA compared with wild-type by western blot analysis (P = .043 vs P = .025) in B cells derived from both spleen and Peyer patches.
Effects of Gna13 on cellular adhesion in vivo and in vitro. (A) Representative histogram of F-actin expression in GC B cells (upper) or FO B cells (lower) from AID-Cre+Gna13 mice. (B) MFI for F-actin expression in AID-Cre+Gna13 mice as measured by flow cytometric analysis in spleen. Dots represent individual animals. Data are representative of 3 experiments. (C) Western blot showing levels of active (guanosine triphosphate–bound) RhoA, total RhoA, and β-actin in B-cell lysates from spleen (left) and Peyer patches (right) of Mb1-Cre+Gna13 mice. Data are representative of 3 experiments. (D) Confocal images of Raji cell line stably transfected with lentiviral constructs: vector (N1), wild-type GNA13, dominant-negative (DN) GNA13-DN (G225A), and constitutively active (CA) GNA13-CA (Q226L). Top row: differential interference contrast; bottom row: phosphotyrosine (pTyr) staining in red. Images are representative examples of 3 experiments. Cells were imaged with a Zeiss LSM510 confocal microscope with a 63×/1.4 NA (numerical aperture) oil immersion lens. (E) Representative histogram and quantitation of pTyr expression by immunofluorescence in Raji cells transfected with constructs described in panel D. Values are quantitated as a percentage of N1 control. Results are representative of 3 experiments. (F) Representative histograms of F-actin expression in Raji cell line expressing the constructs described in panel D. Left: nontreated (N/T); right: in the presence of interleukin-6 (IL-6; 20 ng/mL). MFI, mean fluorescence intensity.
Effects of Gna13 on cellular adhesion in vivo and in vitro. (A) Representative histogram of F-actin expression in GC B cells (upper) or FO B cells (lower) from AID-Cre+Gna13 mice. (B) MFI for F-actin expression in AID-Cre+Gna13 mice as measured by flow cytometric analysis in spleen. Dots represent individual animals. Data are representative of 3 experiments. (C) Western blot showing levels of active (guanosine triphosphate–bound) RhoA, total RhoA, and β-actin in B-cell lysates from spleen (left) and Peyer patches (right) of Mb1-Cre+Gna13 mice. Data are representative of 3 experiments. (D) Confocal images of Raji cell line stably transfected with lentiviral constructs: vector (N1), wild-type GNA13, dominant-negative (DN) GNA13-DN (G225A), and constitutively active (CA) GNA13-CA (Q226L). Top row: differential interference contrast; bottom row: phosphotyrosine (pTyr) staining in red. Images are representative examples of 3 experiments. Cells were imaged with a Zeiss LSM510 confocal microscope with a 63×/1.4 NA (numerical aperture) oil immersion lens. (E) Representative histogram and quantitation of pTyr expression by immunofluorescence in Raji cells transfected with constructs described in panel D. Values are quantitated as a percentage of N1 control. Results are representative of 3 experiments. (F) Representative histograms of F-actin expression in Raji cell line expressing the constructs described in panel D. Left: nontreated (N/T); right: in the presence of interleukin-6 (IL-6; 20 ng/mL). MFI, mean fluorescence intensity.
To determine whether reduced F-actin levels might reflect impaired GC B-cell adhesion in vitro, we assessed focal adhesion formation via confocal microscopy using the human Burkitt-derived Raji cell line, which possesses a nonsynonymous mutation in GNA13.5 We stably infected Raji with lentiviral vectors expressing wild-type GNA13, as well as 2 previously characterized GNA13 mutants,17 G225A and Q226L, which have dominant-negative and constitutively active effects on G-protein activity, respectively. Compared with vector control cells, Raji cells expressing wild-type and Q226L GNA13 have (1) increased numbers of cellular projections evident by differential interference contrast imaging, (2) higher staining intensity of phosphotyrosine, a biochemical feature of focal adhesion sites18 (Figure 2D-E, P = .02); and (3) increased F-actin levels (Figure 2F; supplemental Figure 5A, P = .001), suggesting increased focal adhesion formation. By comparison, G225A GNA13–expressing cells have fewer cellular extensions and reduced phosphotyrosine staining (Figure 2E, P = .0034) and F-actin expression (supplemental Figure 5A, P < .01). These effects are further pronounced by stimulation with interleukin-6, a cytokine that enhances focal adhesion formation in GC B cells16 (Figure 2F, right; supplemental Figure 5A, right). These data support a role for GNA13 in regulating cellular adhesion in GC B cells.
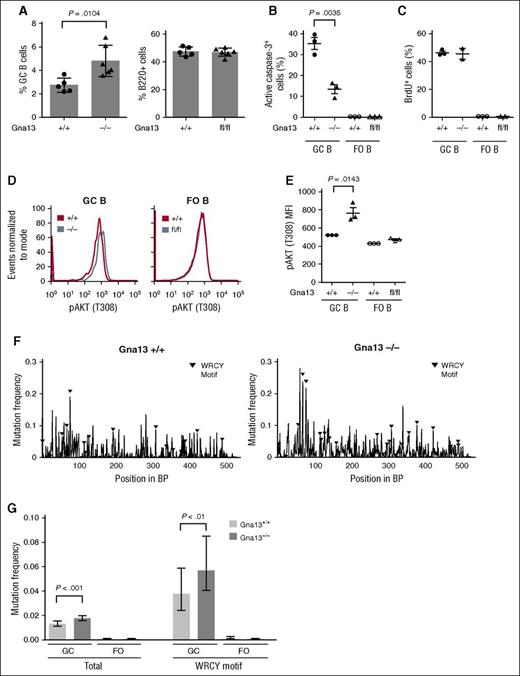
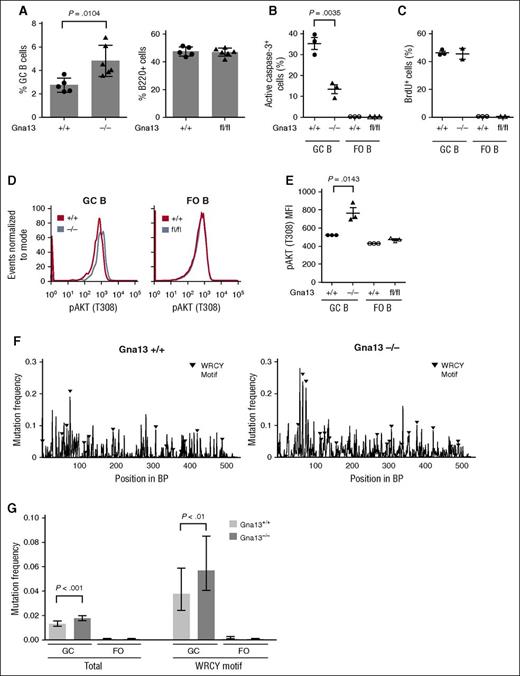
We next evaluated mature B-cell subsets that could potentially be affected by Gna13 deletion in a GC-specific model, including GC B cells, plasma cells, and memory cells. Similar to the previous study, we found that GC B cells were significantly increased in Gna13-null mice (Figure 3A, left, P = .0104 for spleen; supplemental Figure 5B, left, P < .0001 for Peyer patches). By contrast, total B220+ cells remained constant (Figure 3A, right; supplemental Figure 5B, right). We found that despite having elevated numbers of GC B cells, AID-Cre+Gna13 mice had comparable numbers of plasma cells in bone marrow and IgG1 cells (which include memory and class-switched GC B cells) in spleen across genotypes (supplemental Figures 5C and 6). The fact that the GC B-cell expansion is not reflected in a proportional increase in B cells differentiating past the GC stage suggests that GNA13 loss may impair terminal B-cell differentiation, thus restricting the effects of GNA13 loss to GC B cells (and lymphomas).
Gna13 deletion results in expansion of GC B cells, reduced apoptosis, and increased SHM-related mutational burden. (A) Bar graph comparing the GC populations across genotypes in splenic cells isolated from 2-month-old AID-Cre+Gna13 mice. GC cells were calculated as a percentage of total B cells (B220+ cells). Data are representative of 3 experiments. Fluorescence-activated cell sorting analysis of active caspase-3 (B) and 5-bromo-2′-deoxyuridine (BrdU) (C) in B cells isolated from spleen of 3-month-old AID-Cre+Gna13 mice. In each graph, GC cells are depicted on the left, with FO cells on the right as a control. Dots represent individual mice. Data are representative of 3 experiments. (D) Representative histogram of pAKT (T308) expression in GC and FO B cells from spleen of AID-Cre+Gna13 cohort mice. Wild-type and null GC B cells are depicted by blue and red traces, respectively. (E) MFI for pAKT (T308) expression in AID-Cre+Gna13 cohort mice as measured by flow cytometry in spleen. Data are representative of 3 experiments. (F-G) SHM analysis of the JH4 intronic region of the VH immunoglobulin locus. Data are depicted as mutational frequency per base across an amplified segment of the JH4 intronic region of GC B cells (F) and quantitated in bar-graph form (G), along with FO controls. WCRY/RGYW hotspot motifs are depicted with black triangles (F) and quantitated separately (G, right). Data reflect an average depth of ∼7500 reads per genotype from GC B cells and from FO B cells isolated from Peyer patches of wild-type or null AID-Cre+Gna13 animals.
Gna13 deletion results in expansion of GC B cells, reduced apoptosis, and increased SHM-related mutational burden. (A) Bar graph comparing the GC populations across genotypes in splenic cells isolated from 2-month-old AID-Cre+Gna13 mice. GC cells were calculated as a percentage of total B cells (B220+ cells). Data are representative of 3 experiments. Fluorescence-activated cell sorting analysis of active caspase-3 (B) and 5-bromo-2′-deoxyuridine (BrdU) (C) in B cells isolated from spleen of 3-month-old AID-Cre+Gna13 mice. In each graph, GC cells are depicted on the left, with FO cells on the right as a control. Dots represent individual mice. Data are representative of 3 experiments. (D) Representative histogram of pAKT (T308) expression in GC and FO B cells from spleen of AID-Cre+Gna13 cohort mice. Wild-type and null GC B cells are depicted by blue and red traces, respectively. (E) MFI for pAKT (T308) expression in AID-Cre+Gna13 cohort mice as measured by flow cytometry in spleen. Data are representative of 3 experiments. (F-G) SHM analysis of the JH4 intronic region of the VH immunoglobulin locus. Data are depicted as mutational frequency per base across an amplified segment of the JH4 intronic region of GC B cells (F) and quantitated in bar-graph form (G), along with FO controls. WCRY/RGYW hotspot motifs are depicted with black triangles (F) and quantitated separately (G, right). Data reflect an average depth of ∼7500 reads per genotype from GC B cells and from FO B cells isolated from Peyer patches of wild-type or null AID-Cre+Gna13 animals.
We next addressed whether GC expansion in the GNA13-deficient state was due to increased cellular proliferation or decreased apoptosis. Gna13-null GC B cells demonstrated reduced activated caspase-3 relative to wild-type GC cells (Figure 3B, P = .0035). By contrast, Gna13-null GC B cells demonstrated 5-bromo-2′-deoxyuridine incorporation indistinguishable from controls (Figure 3C). These data imply that resistance to apoptosis, rather than increased proliferation, accounts for the increase in GC B-cell number observed in the Gna13-deleted state. AKT activation is associated with resistance to programmed cell death in a variety of tumor types. Work by Muppidi et al demonstrated increased pAKT in the Mb1-Cre+Gna13–null mouse,10 an effect we found reproducible in GC B cells in the AID-Cre model (Figure 3D-E, P = .0143 for spleen; supplemental Figure 5D, P = .0365 for Peyer patches).
SHM occurs almost exclusively during the transient GC B-cell differentiation state.19 Off-target SHM has been reported to occur in certain human lymphomas20 and normal mouse GC B cells deficient in DNA repair.21 Thus, prolonged exposure to the effects of SHM could mutagenize other genes throughout the genome. If impaired apoptosis and potentially delayed differentiation of GC B cells observed in GNA13 deficiency prolong the duration of SHM, one would expect to observe an increased mutational burden in the immunoglobulin loci of affected cells. We thus measured mutation frequencies within the JH4 region of the VH locus of GC B cells isolated from Peyer patches of AID-Cre+Gna13 mice using next-generation sequencing. As expected, wild-type GC B cells have a higher mutation frequency compared with that of FO B cells, which have not undergone SHM (Figure 3G, GC B cells 26.4-fold higher than FO B cells; supplemental Figure 7A). Other hallmarks of SHM, such as an enhanced A:T ratio on the nontemplate strand, were also similar to previously reported values in GC B cells compared with FO controls (supplemental Figure 7B).21 We found that Gna13-null GC B cells had a 1.3-fold higher mutation frequency within the assessed JH4 intronic region compared with wild-type GC B cells (Figure 3F-G, 0.0176 compared with 0.0132, respectively, P = 2.64 × 10−8). This finding was also evident in the AID hotspot motifs WRCY and RGYW (0.0568 and 0.0376, respectively, 1.5-fold higher in null compared with wild-type GC B cells, P = .0057) and was reproducible in GC B cells isolated from Peyer patches of Mb1-Cre+Gna13 cohort mice (supplemental Figure 7C). This consequence of Gna13 status was comparable in magnitude to effects reported for DNA repair–deficient mouse strains, including Polη−/− and MSH6−/− models.11,22
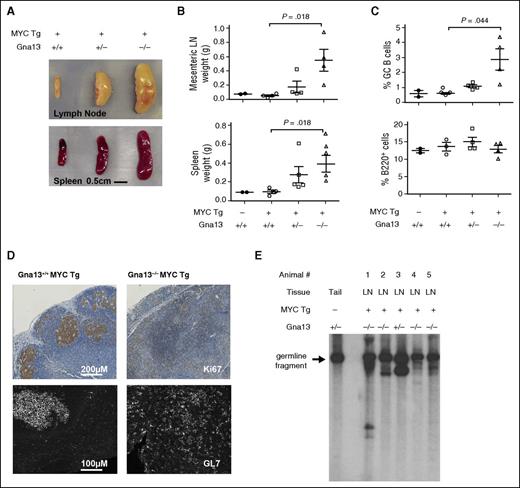
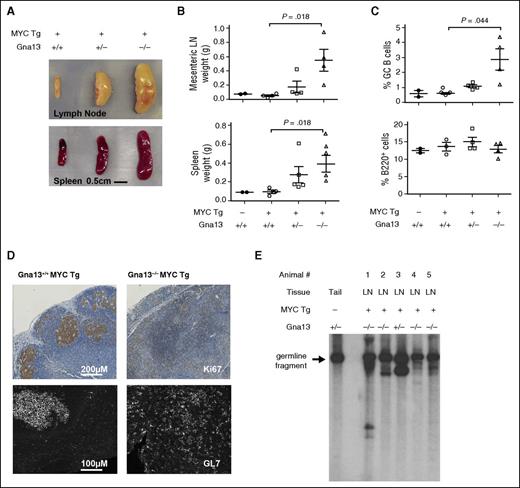
Because genetic alterations in MYC frequently co-occur with GNA13 mutations in GC-derived B-cell lymphomas,4,5 we modeled GNA13 loss and MYC overexpression specifically in GC B cells by crossing the AID-Cre+Gna13 knockout strain with R26StopFLMYC transgenic mice.23,24 Animals were immunized with intraperitoneal sheep red blood cell injection at ∼9 weeks of age and euthanized at ∼30 weeks (supplemental Figure 8A). At the time of necropsy, 4 of 4 animals null for Gna13 and expressing the MYC transgene in GC B cells had developed splenomegaly and lymphadenopathy, which were predominantly abdominal. We also noted disseminated lymphadenopathy (cervical, inguinal, and lumbar nodes) consistent with metastasis (Figure 4A-B, P = .018 for wild-type compared with null mice for both mesenteric lymph node and spleen). Two of 4 animals also had evidence of thymic enlargement. Four of 5 Gna13 heterozygous mice expressing the MYC transgene exhibited mild organomegaly (Figure 4A-B, not significant). The remaining heterozygous Gna13 mouse was euthanized for respiratory distress and weight loss, and was found to have a large pleural tumor in addition to lymphadenopathy and splenomegaly. By contrast, Gna13 wild-type MYC transgenic mice (n = 4) exhibited spleen and lymph node weights indistinguishable from nontransgenic controls, consistent with the previous characterization of this MYC transgenic model.24 Quantitative PCR using messenger RNA isolated from spleens of these Gna13 MYC transgenic cohort animals demonstrated MYC overexpression and Gna13 loss in the appropriate genotypes (supplemental Figure 8B). For Gna13-null animals, organomegaly was associated with an increase in the proportion of B cells expressing GC markers (Figure 4C, P = .044; supplemental Figure 8C). Immunohistochemical characterization of Ki67 expression (Figure 4D, top) demonstrates effacement of the GC structure in sections of mesenteric lymph node from a representative Gna13-null MYC transgenic animal compared with control. Immunofluorescent detection of GL7 on corresponding splenic sections demonstrates Gna13-mutant MYC-transgenic GC B cells scattered throughout the tissue, with disrupted architectural organization. This finding may represent a continued evolution of the phenotype observed in Gna13-null mice without MYC transgene, in which GL7+ cells appear outside GC borders in increasing numbers (Figure 1B). To determine whether the observed phenotype resulted from a clonal B-cell expansion, we performed Southern blot analysis assessing immunoglobulin heavy chain (IgH) locus rearrangement. The arrow in Figure 4E indicates the 6.5-kb germ-line fragment present in normal tail DNA, whereas in tumor samples, additional bands are present, representing clonally rearranged IgH alleles in affected animals, including the Gna13 heterozygous animal with thoracic disease (animal 3). The germ-line fragment remains present in all the tested samples, reflecting the relative contribution of non-B cells to the affected tissue. Taken together, these findings are consistent with mature B-cell lymphoma in these animals.
Characterization of B-cell lymphomas arising from AID-Cre+R26StopFLMYC Gna13–deficient mice. (A) Photograph of spleens and mesenteric lymph nodes from representative MYC+Gna13 genotypes. (B) Comparison of the weights of spleens and mesenteric lymph nodes (LN) from MYC−Gna13 wild-type (n = 2) mice and MYC+Gna13 wild-type (n = 4), heterozygous (n = 5 for spleen, 4 for mesenteric), and null (n = 4) mice. (C) Graph of GC B-cell and total B-cell populations in AID-Cre+MYC+Gna13 mice across genotypes. (D) Immunohistochemical staining of proliferation marker Ki67 (top row) or immunofluorescent staining of GC marker GL7 (bottom row) in FFPE sections of mouse lymph node from AID-Cre+MYC+Gna13 mice. (E) Tumor clonality as determined by IgH rearrangement patterns. Southern blot analysis was performed using a heavy-chain joining-region probe to detect changes in fragment patterns in EcoRI digested genomic DNA extracted from mouse tissue. The arrow indicates the 6.5-kb germ-line DNA fragment present in wild-type mouse tail DNA, and its presence in all samples reflects the relative contribution of non-B cells to a given sample. Tg, transgenic.
Characterization of B-cell lymphomas arising from AID-Cre+R26StopFLMYC Gna13–deficient mice. (A) Photograph of spleens and mesenteric lymph nodes from representative MYC+Gna13 genotypes. (B) Comparison of the weights of spleens and mesenteric lymph nodes (LN) from MYC−Gna13 wild-type (n = 2) mice and MYC+Gna13 wild-type (n = 4), heterozygous (n = 5 for spleen, 4 for mesenteric), and null (n = 4) mice. (C) Graph of GC B-cell and total B-cell populations in AID-Cre+MYC+Gna13 mice across genotypes. (D) Immunohistochemical staining of proliferation marker Ki67 (top row) or immunofluorescent staining of GC marker GL7 (bottom row) in FFPE sections of mouse lymph node from AID-Cre+MYC+Gna13 mice. (E) Tumor clonality as determined by IgH rearrangement patterns. Southern blot analysis was performed using a heavy-chain joining-region probe to detect changes in fragment patterns in EcoRI digested genomic DNA extracted from mouse tissue. The arrow indicates the 6.5-kb germ-line DNA fragment present in wild-type mouse tail DNA, and its presence in all samples reflects the relative contribution of non-B cells to a given sample. Tg, transgenic.
Discussion
In this study, we have modeled Gna13 deletion specifically in GC B cells to understand how the genetic alterations in GNA13 commonly observed in human GC B-cell lymphomas might contribute to the pathogenesis of tumors arising from this unique niche. Similar to results reported by Muppidi et al10 using Mb1-Cre+Gna13 conditional knockout mice, we found that homozygous Gna13 deficiency is associated with expansion of the GC population beyond the GC boundaries and with elevated levels of pAKT within GC B cells. One limitation of the Muppidi et al10 study is that the Mb1-Cre transgene leads to Gna13 deletion from the pre/pro B-cell stage, which leaves the possibility of GNA13-related defects in B cell development and confounding phenotypes in non-GC mature B-cell subtypes. Our confirmation of these findings in the AID-Cre model suggests that the phenotype is dominated by events occurring at the GC stage of B-cell development, likely reflecting that Gna13 expression is largely restricted to the GC B-cell differentiation stage within the B-cell lineage.25
In the present study, we further found that Gna13 deletion in GC B cells is also associated with altered zonal population ratios within the GC in vivo and with impaired cellular adhesion in vitro. Impaired cell death of GC B cells was also found to be associated with an increased mutational burden in the IgH locus, suggesting prolonged exposure to AID. Lastly, we report that Gna13 deletion confers a strong susceptibility to lymphoma in a CRE-activated MYC transgenic model. This may be due to the combined effect of MYC-driven proliferation and impaired cell death conferred by GNA13 loss. A similar propensity for lymphomagenesis has been described in mice expressing a constitutively active form of phosphatidylinositol 3-kinase, P110*, in the context of a similar GC-restricted MYC transgenic model, with tumors developing at ∼250 days of age, a timeline similar to our model.24 Analogous to Gna13 deficiency, P110* expression leads to elevated levels of pAKT and protection against apoptosis. Synergy between enhanced proliferation and reduced cell death has also been well described in human double-hit lymphoma, in which simultaneous MYC and BCL2 overexpression leads to a particularly aggressive disease phenotype.26
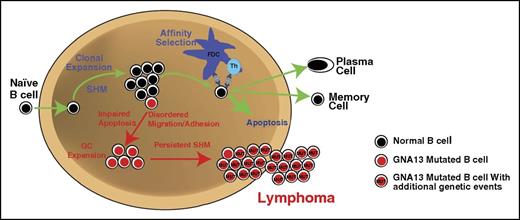
Figure 5 is a proposed model of the mechanism by which GNA13 mutations may promote lymphoma in the GC, taking into account data presented here and by Muppidi et al.10 Inactivating GNA13 mutations promote altered GC B-cell migration within and beyond the GC, as well as impaired cellular adhesion, resulting in cells that may have a reduced ability to establish interactions with GC helper cells. Under normal conditions, a GC cell unable to form these helper cell interactions, either due to GC exit or ineffective cellular adhesion, would undergo apoptosis. However, GNA13-mutated GC B cells are resistant to programmed cell death. By uncoupling affinity selection from survival, GNA13-mutated cells can persist in the GC B cell differentiation state. This would permit ongoing SHM, the off-target effects of which may result in the accumulation of additional driver mutations that promote lymphoma.
Proposed mechanism whereby GNA13 loss promotes lymphomagenesis within the GC niche. In normal circumstances, GC B cells follow the path depicted with green arrows. Mutations in GNA13 lead to disordered migration and impaired apoptosis. Affected cells likely do not require prosurvival signals from T helper (Th) cells to persist. GC persistence may promote the accumulation of additional mutations through ongoing SHM. Over time, accumulation of driver mutations in persistent GC cells may promote lymphoma. FDC, follicular dendritic cell.
Proposed mechanism whereby GNA13 loss promotes lymphomagenesis within the GC niche. In normal circumstances, GC B cells follow the path depicted with green arrows. Mutations in GNA13 lead to disordered migration and impaired apoptosis. Affected cells likely do not require prosurvival signals from T helper (Th) cells to persist. GC persistence may promote the accumulation of additional mutations through ongoing SHM. Over time, accumulation of driver mutations in persistent GC cells may promote lymphoma. FDC, follicular dendritic cell.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute grants, the Leukemia Lymphoma Society, and the V Foundation (S.S.D.).
Authorship
Contribution: J.A.H., A.N., and R.E.R. designed and executed the experiments, interpreted the data, and were primary contributors to the text and figures; A.B.M. and N.S.D. executed the experiments, interpreted the data, and contributed to the text and figures; T.J.T., J.R.S., J.Z., C.L., J.-T.C., and K.A.W. executed the experiments; M.E.M. contributed to the experimental design; J.D. contributed to the statistical analysis; X.J. executed the experiments and contributed to the experimental design and analysis; N.W., S.O., S.A.K.R., P.J.C., and I.S.L. contributed to the experimental design and analysis; and S.S.D. contributed to the experimental design, data interpretation, and text and figure editing, and was senior principal investigator on the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandeep S. Dave, Duke University, 101 Science Dr, Box 3382, Durham, NC 27710; e-mail: sandeep.dave@duke.edu.
References
Author notes
J.A.H., A.N., and R.E.R. contributed equally to this work.