Abstract
Vaccination guidelines for recipients of blood and marrow transplantation (BMT) have been published by 3 major societies: American Blood and Marrow Transplantation, European Group of Blood and Marrow Transplantation, and Infectious Disease Society of America. Despite these extensive review articles, clinicians caring for BMT recipients continue to field frequently asked questions (FAQs) regarding the “who, when, and how” of feasible and effective posttransplant vaccination, frequently in the absence of adequate data. This may reflect discomfort with a “one size fits all” policy that makes no adjustments for different posttransplant clinical scenarios. Existing guidelines also lack practical dose clarifications when administering vaccines to patients who differ by age, underlying diagnosis, or amount of immunosuppressive therapy. Frequently, little or conflicting guidance is given regarding age-related schedules for certain vaccines (eg, meningococcal; tetanus toxoid, reduced diphtheria toxoid, and reduced acellular pertussis; and human papillomavirus vaccines) in addition to time posttransplant or other factors. FAQs and their answers form the body of this article and are shared with readers as a concise practical review, with the intent to facilitate good clinical practice.
Introduction
Three major societies (American Blood and Marrow Transplantation, European Group of Blood and Marrow Transplantation, and Infectious Disease Society of America) have published vaccination guidelines separately1 or as part of broad practice guidelines for preventing infectious complications among blood and marrow transplantation (BMT) recipients or other immunocompromised hosts.2,3 Despite these extensive guidelines, only 17% of anonymously polled participants at a 2015 American Blood and Marrow Transplantation practice guidelines session were aligned with the overarching societal recommendation to begin vaccinations at 6 months for patients without graft-versus-host disease (GVHD); 64% of participants requested basic data about the patient’s numeric immune reconstitution (IR), which is not recommended by current guidelines. This may reflect a discomfort resulting from “one-size fits all” recommendations, especially when Infectious Disease Society of America states upfront that the “evidence is often limited.” Existing guidelines make little adjustment for whether BMT was for primary immunodeficiency disease or other factors that most providers consider when beginning and dosing vaccines. Other factors generally reviewed in addition to the time after transplant are levels of numeric or functional IR, the intensity of recent or ongoing immunosuppressive therapy (IST), and age-related schedules for certain vaccines (eg, meningococcal, pneumococcal, Tdap [tetanus toxoid, reduced diphtheria toxoid, and reduced acellular pertussis], and human papillomavirus [HPV]).
This “How I Treat” arose from our need for pragmatic institutional vaccination standards while recognizing the dearth of existing data to address many relevant practicalities. The goal for this article was to discuss vaccination clinical cases (Figure 1) and our “top 20” frequently asked questions (FAQs) about post-BMT vaccination. Supplementary FAQs (sFAQs) that did not make the top 20 and those about less common vaccines are found in the supplemental Frequently Asked Questions (available on the Blood Web site). Questions came from clinical providers via phone, e-mail, or as “curbside consults,” which often began with: “Do you mind if I just ask you a vaccine question?” We share our answers to these FAQs as a concise and pragmatic review; the intent is to facilitate good clinical practice in patients of all ages. We also provide an autofilled Excel immunization schedule table for our recommended BMT approach to early or standard vaccination (supplemental Table 1).
Posttransplant vaccination case histories with individualized and autopopulated vaccination schedules shown for 3 different cases (white boxes). Case 1: a 19-year-old adolescent underwent allogeneic BMT on March 15, 2016, for leukemia, did not develop chronic GVHD, and on September 15, 2016, at 6 months posttransplant had an unsupported immunoglobulin G (IgG) level of 780 mg/dL. She was varicella zoster virus (VZV) seropositive pretransplant. (A) Her schedule for beginning inactivated vaccines at 6 months posttransplant highlights age-related considerations for HPV vaccine (FAQ 10) as well as when to offer conjugated quadrivalent and group B meningococcal vaccines (FAQ 14). (B) Live vaccines are considered at 2 years posttransplant. Case 2: a 59-year-old man underwent nonmyeloblative allogeneic transplant for chronic lymphocytic leukemia on October 14, 2015, but received rituximab early after transplant, and thus early vaccination at 6 months was not considered appropriate (FAQ 1; Figure 2). He was VZV-seropositive pretransplant. Posttransplant, his last dose of intravenous immunoglobulin (IVIG) was at 11 months, when his B-cell count was 80/μL and serum IgG 665 mg/dL. (C) On October 14, 2016, he began
Posttransplant vaccination case histories with individualized and autopopulated vaccination schedules shown for 3 different cases (white boxes). Case 1: a 19-year-old adolescent underwent allogeneic BMT on March 15, 2016, for leukemia, did not develop chronic GVHD, and on September 15, 2016, at 6 months posttransplant had an unsupported immunoglobulin G (IgG) level of 780 mg/dL. She was varicella zoster virus (VZV) seropositive pretransplant. (A) Her schedule for beginning inactivated vaccines at 6 months posttransplant highlights age-related considerations for HPV vaccine (FAQ 10) as well as when to offer conjugated quadrivalent and group B meningococcal vaccines (FAQ 14). (B) Live vaccines are considered at 2 years posttransplant. Case 2: a 59-year-old man underwent nonmyeloblative allogeneic transplant for chronic lymphocytic leukemia on October 14, 2015, but received rituximab early after transplant, and thus early vaccination at 6 months was not considered appropriate (FAQ 1; Figure 2). He was VZV-seropositive pretransplant. Posttransplant, his last dose of intravenous immunoglobulin (IVIG) was at 11 months, when his B-cell count was 80/μL and serum IgG 665 mg/dL. (C) On October 14, 2016, he began
Top 20 FAQs
General
FAQ 1: when should vaccinations begin for the typical BMT recipient?
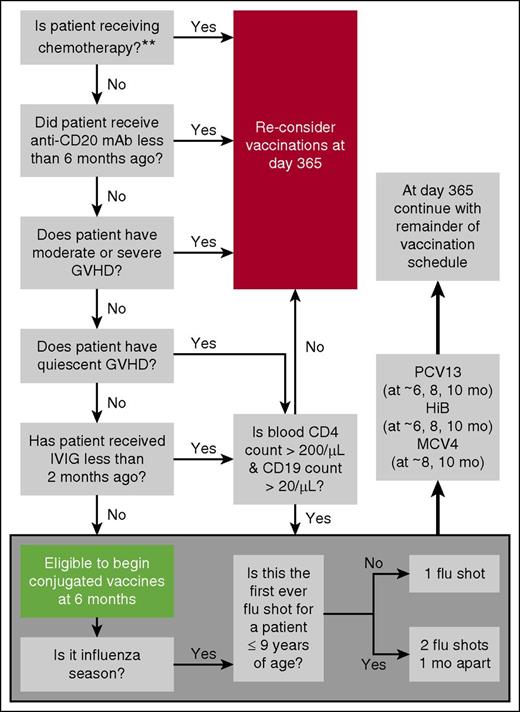
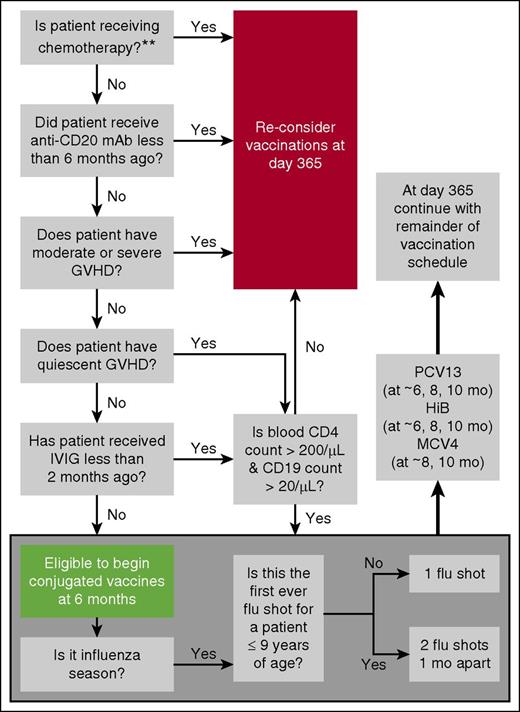
Consensus guidelines recommend initiating vaccinations at 3 to 6 months after BMT noting that this lacks prospective validation.1-5 Given the variable tempo of IR (see sFAQ 1), particularly in patients transplanted for primary immunodeficiency diseases (see FAQ 4), the negative impact of moderate to severe GVHD on IR, and potential use of in vivo T- or B-cell depleting therapies, we prefer to select candidates for “early vaccination” at 6 months based on favorable responses to a 6-question algorithm (Figure 2). Any unfavorable response triggers vaccine deferral (except for flu shots) until at least 1 year posttransplant. Our approach takes into consideration that vaccine efficacy depends on at least partial reconstitution of adaptive immunity.
Screening questions to determine early vaccination candidacy. Note: B-cell numbers are low in the first 1 to 2 months and normalize during months 3 to 12. B-cell recovery is delayed by at least 6 months after anti-B-cell antibody therapy. Antigen-specific responses are impaired also because of limited capacity to undergo somatic mutation and isotype switch during the first year. Normalization of IgA levels can be indicative of isotype switching and is unaffected by IVIG replacement therapy. CD4 counts are generally <200/μL during the first 3 months. Thereafter, recovery is highly variable, generally >200/μL by 6 to 9 months if age <18 years and no chronic GVHD. Adults with chronic GVHD may take >2 years. For these reasons, some institutions defer vaccination until the peripheral CD4 count is >200/μL and the CD19 (B-cell) count is >20 /μL. Most circulating T cells at year 1 (especially in adults) are memory/effector T cells derived from infused T cells. These cells can respond to antigens encountered by the donor pre-BMT. Naïve T cells that respond to neoantigens are generated only at 6 to 12 months (earlier in young children, later in old adults). Only limited data exist for the settings of unrelated cord blood and haploidentical HCT or after reduced intensity regimens, and so, for the sake of simplicity, the algorithm does not make further adjustments on these bases. The double asterisk (**) indicates that which agents are sufficiently immunosuppressive to prevent effective vaccination has not been studied. Other than the seasonal flu shot, most vaccinations are avoided when BMT recipients are receiving azacytidine, lenalidomide, or rituximab. We tend to still administer vaccines if patients have little or no chronic GVHD and are on kinase inhibitors (eg, imatinb or sorafenib).
Screening questions to determine early vaccination candidacy. Note: B-cell numbers are low in the first 1 to 2 months and normalize during months 3 to 12. B-cell recovery is delayed by at least 6 months after anti-B-cell antibody therapy. Antigen-specific responses are impaired also because of limited capacity to undergo somatic mutation and isotype switch during the first year. Normalization of IgA levels can be indicative of isotype switching and is unaffected by IVIG replacement therapy. CD4 counts are generally <200/μL during the first 3 months. Thereafter, recovery is highly variable, generally >200/μL by 6 to 9 months if age <18 years and no chronic GVHD. Adults with chronic GVHD may take >2 years. For these reasons, some institutions defer vaccination until the peripheral CD4 count is >200/μL and the CD19 (B-cell) count is >20 /μL. Most circulating T cells at year 1 (especially in adults) are memory/effector T cells derived from infused T cells. These cells can respond to antigens encountered by the donor pre-BMT. Naïve T cells that respond to neoantigens are generated only at 6 to 12 months (earlier in young children, later in old adults). Only limited data exist for the settings of unrelated cord blood and haploidentical HCT or after reduced intensity regimens, and so, for the sake of simplicity, the algorithm does not make further adjustments on these bases. The double asterisk (**) indicates that which agents are sufficiently immunosuppressive to prevent effective vaccination has not been studied. Other than the seasonal flu shot, most vaccinations are avoided when BMT recipients are receiving azacytidine, lenalidomide, or rituximab. We tend to still administer vaccines if patients have little or no chronic GVHD and are on kinase inhibitors (eg, imatinb or sorafenib).
FAQ 2: should BMT recipients be vaccinated if they already have protective titers?
Yes. Antibody titers to vaccine-preventable diseases decline after autologous (see sFAQ 2) or allogeneic BMT despite the fact that most BMT recipients were vaccinated earlier in life.6 Clinical relevance of declining titers is not immediately apparent because the number of vaccine-preventable diseases reported among BMT recipients is limited. Nonetheless, diseases potentially prevented by vaccines still pose increased risks for BMT recipients until immunity is fully restored. Therefore, with exception of viruses exhibiting latency outside the hematopoietic system (eg, varicella), BMT recipients should be revaccinated against pathogens contained in childhood primary immunization schedules.
FAQ 3: is vaccination appropriate for patients receiving immunoglobulin therapy?
For inactivated vaccines, recently administered IgG products do not inhibit immune responses (see sFAQ 7).7 However, we do not recommend early vaccination when patients are receiving IVIG replacement therapy because IVIG therapy is a surrogate marker for delayed IR (Figure 2). If a decision is made to vaccinate at 12 months posttransplant, then delaying vaccination after IgG products might create a missed opportunity to vaccinate a patient who is seen infrequently, and immunization in this scenario is therefore recommended.
In contrast, the ACIP recommends deferring live MMR and VZV vaccination no sooner than 3 to 11 months after receiving the last IgG-containing blood products. This allows sufficient degradation of potential antibodies that could interfere with viral replication, the latter being essential to effective live virus vaccination.8 Other criteria need to be considered for live virus vaccination in the transplant recipient (FAQs 15, 19 and also sFAQs 30, 35-40).
FAQ 4: are recipients of allogeneic BMT for primary immune deficiency vaccinated differently than recipients transplanted for other diseases?
Yes. Because B-cell IR has been highly variable after BMT in patients with immunodeficiencies, vaccination is delayed until there is robust evidence of functional B-cell recovery. Bacteriophage testing may be done when IST is discontinued unless there is a history of anti-CD20 antibody therapy or poor donor B-cell engraftment,9 but results of bacteriophage testing are not the major factor in deciding when to begin vaccination. Except for seasonal flu shots (see FAQ 11), routine posttransplant vaccinations are not considered until at least 1 year after BMT and only if the following 3 criteria are satisfied:
Patient is without infections in the past 6 months, and so it is reasonable to attempt a 3-month trial off IgG replacement therapy and
Low community prevalence of influenza, respiratory syncytial virus, human metapneumovirus, or parainfluenza is present during the planned trial off IgG therapy and
All the following laboratory criteria are met:
(a) Trough IgG >600 mg/dL on standard IgG dosing, which suggests numeric IgG reconstitution.
(b) Detectable serum IgA (>6 mg/dL) suggesting ability to “Ig class switch.”
(c) Donor B cells >200/μL as determined by percent donor B-cell chimerism multiplied by the total absolute B-cell count. This threshold is arbitrarily set 1-log higher than for nonprimary immunodeficiencies diseases.
(d) Donor CD4 cells 200/μL as determined by percent donor CD4 chimerism multiplied by the total absolute CD4 T-cell count.
Generally, the last criterion is addressed after the first 2 criteria are met by ensuring that quantitative immunoglobulin testing is timed with the expected IgG nadir following the last dose of IgG therapy. When all 3 criteria are satisfied, IgG therapy is withheld for 12 consecutive weeks. We begin (week 0) by giving 1 dose each of PCV13, Hib, DTaP (or Tdap age >10), and hepatitis B virus (HBV). Combination vaccines are preferred in children to limit the number of injections. During weeks 6 to 8, we repeat the series given at week 0. At week 12, we check antibody titers to Hib, 23-serotypes of pneumococcus (expecting only a response to PCV13 serotypes), tetanus toxoid, and HBV surface antibody. Responders then remain off IgG therapy and proceed with a third dose of each of these vaccines. Patients then receive a standard series of meningococcal, HPV, hepatitis A virus (HAV), and inactivated polio vaccines. Alternatively, if vaccine response is inadequate, IgG therapy is resumed and further vaccination will be deferred.
FAQ 5: why use conjugated vaccines including Hib, PCV13, and MCV4 to initiate early vaccinations?
Because of impaired opsonization, B-cell-deficient patients are highly susceptible to encapsulated bacteria (Hib, Neisseria meningitidis, Streptococcus pneumonia). BMT recipients without a spleen or with functional asplenia because of chronic GVHD are similarly susceptible (see sFAQ 3). Young children are most at risk because of limited capacity to make antibodies to polysaccharide capsules while responding more favorably to protein conjugate vaccines. Similarly, BMT recipients respond to conjugate vaccines as early as 3 months posttransplant and at higher rates than polysaccharide vaccines.4,5
FAQ 6: can household contacts and health care workers who interact with immunocompromised hosts be administered live attenuated vaccines?
Available data support routine administration of such individuals with injectable live attenuated MMR or varicella vaccine if otherwise age appropriate. However, intranasal live attenuated influenza vaccine (LAIV) should not be given to family members of seriously immunocompromised hosts, such as those requiring protective isolation, because immunocompetent children can shed LAIV for several days. That said, estimated secondary transmission rates of LAIV among immunocompetent hosts are likely <0.001%; LAIV spread from healthy individuals to an immunocompromised patients has not been reported. If a health care worker caring for individuals in protective isolation is given LAIV, a 7-day furlough is advised.10 Live attenuated oral polio vaccine that is still available in some non-US countries should not be used and if inadvertently given to a household contact, a 4- to 6-week furlough is advised.
DTaP/Tdap
Tetanus toxoid revaccination is relevant given the ubiquitous environmental exposure to tetanus and the seriousness of disease because of tetanus toxin. Although diphtheria is essentially eradicated, ongoing vaccination is critical for herd immunity, and pertussis outbreaks have been prevalent in states where herd immunity has waned. It is important to remember that in the prevaccination era (1920s to 1940s), annual US death rates for pertussis were 5000 to 10 000.
FAQ 7: should I administer DTaP or Tdap?
Consensus guidelines favor 3 doses of DTaP regardless of age, although this policy is inconsistent with US recommendations because DTaP is only licensed for <7 years of age. Without data to support DTaP being safe and more effective than Tdap for BMT recipients aged ≥7 years, the consensus approach needs to be weighed by individual centers. The argument to offer DTaP and not Tdap for older BMT recipients is that 10-fold higher doses of diphtheria and pertussis toxoids in DTaP should elicit better antibody responses. This argument aligns with the fact that early vaccination could include patients who remain on IST (FAQs 1, 4). For reference, the strength of the recommendation for 3 doses of pediatric DTaP vaccine in <7 year olds is rated by IDSA as “strong” with only “low” quality supporting evidence. In contrast, the same 3-dose DTaP series in ≥7 year olds (ie, US Food and Drug Administration [FDA] nonapproved use) is rated with a “weak” recommendation and “very low” quality of evidence. Finally, the FDA/ACIP-approved 3-dose series for ≥7 year olds (Tdap/Td/Td) also carries a “weak” recommendation with “low” rather than “very low” quality evidence. It is unclear whether DTaP side effects are related to higher doses of “D” or “aP” antigens. One study in healthy adults demonstrated that the aP vaccine was safe and relatively nonreactogenic even at high doses.11 Currently, compliance with FDA licensure limits US practice to the potentially inferior Tdap vaccine. If we restrict the use of a 3-shot DTaP series to <7 year olds, the testable question remains whether 3 doses of Tdap would be better than Tdap/Td/Td for those over 7 years of age.
Hib
Before Hib conjugate vaccines were introduced, Hib was the leading US cause of bacterial meningitis among children <5 years old. Hib was also a major cause of other life-threatening infections including pneumonia, epiglottis, bacteremia, and others (see FAQ 5 and sFAQs 3, 15).
HAV and HBV
HAV infection can be serious and prolonged, particularly in older children, teens, and adults. A 2-shot series is advised to provide durable protective immunity.
Routine US childhood HBV vaccination after 1990 contributed to a >80% decline but incomplete eradication of new acute HBV cases. Thirty percent to 50% of infected persons aged 5 years and older have initial signs and symptoms for up to 6 months. Fatality rates for Centers for Disease Control–reported acute cases are 0.5% to 1%, and disease is more severe among older adults. Although 95% of adults recover and clear the infection, 90% of infants (and up to 50% aged 1-5 years) will remain chronically infected. Fatality rates for chronic HBV-induced cirrhosis or hepatocellular carcinoma are 25% if infection is acquired during childhood and 15% if infected at older ages. There is no treatment of acute infection; chronic infection involves chronic antiviral therapy and regular monitoring to screen for liver damage and cancer. Not surprisingly, broad ACIP HBV vaccine recommendations include immunocompromised individuals.
FAQ 8: how should HAV and HBV vaccines be dosed in BMT recipients?
HAV vaccination does not differ for immunocompetent and immunocompromised individuals. However, because HBV vaccine antigen doses need to be higher for adult hemodialysis patients to induce protective antibody, the Centers for Disease Control suggests that higher doses or additional doses of HBV vaccine might also be necessary for other immune-compromised individuals. We interpret this to include BMT recipients at least until they reach the milestone of being 6 months off all IST. The nuances of age-related HAV and HBV vaccine dosing and use of combination vaccines are detailed in Tables 1 and 2 (see also sFAQs 16-22).
HPV
FAQ 9: why is vaccination against HPV advised for young BMT recipients?
HPV infection is caused by the most common sexually transmitted virus in the United States, with >50% of sexually active individuals infected (mostly asymptomatically) during their lifetimes. HPV is responsible for cervical, vaginal, and vulvar cancer in women and is the second leading cause of cancer deaths in women worldwide. HPV is also responsible for genital warts and anal cancers in both genders; genital warts can be serious in immunocompromised individuals.
FAQ 10: which HPV vaccine should be administered after BMT?
Among 3 currently available HPV vaccines (4vHPV, 2vHPV, 9vHPV), 9-valent HPV has the potential to prevent ∼90% of cervical, vulvar, vaginal, and anal cancers. It protects against cervical cancer and genital warts. 2vHPV protects against serotypes that are responsible for ∼70% of cervical cancer but does not contain HPV types 6 and 11 that are responsible for more than 90% of genital warts. ACIP currently advises vaccination with either 4vHPV or 9vHPV in immunocompromised persons age 9 through 26 years (see sFAQ 23).12 Although 2 doses may be sufficient for 4vHPV vaccine in healthy individuals,13 absence of data in BMT leads us to advise 3 doses be recommended.
Influenza vaccine
Although annual influenza vaccination is a “universal” ACIP recommendation for all individuals age ≥6 months of age, it is especially important for those at high risk for serious flu complications: children aged <5 years or adults aged >65 years and those with “weakened immune systems” including BMT recipients, especially when complicated by chronic lung disease. Even if flu vaccination does not prevent infection with influenza, studies show that it can reduce the risk for hospitalizations and deaths attributable to flu, especially relevant to patients with chronic GVHD because of their risk for bronchiolitis obliterans syndrome and general immune compromise. A variety of flu vaccines are available (sFAQ 24-28).
FAQ 11: how soon after BMT can I give the flu shot?
Administer at ≥6 months post-BMT regardless of conditioning regimen or BMT type. During community outbreaks flu vaccine may be given at 3 to 4 months post-BMT, in which case a second dose is given 1 month later.14
FAQ 12: is post-BMT flu vaccination different for children compared with adults?
Yes, but only for children aged ≥6 months and <9 years who never had flu vaccine posttransplant; these children need 2 flu shots given ≥1 month apart. One study has not demonstrated further benefit from 2 doses in pediatric BMT recipients who have not received influenza vaccine posttransplant, and therefore, just 1 dose is given annually thereafter.15 For healthy family members aged 2 to 8 years, live attenuated influenza vaccine (FluMist, MedImmune) or the inactivated flu shot is considered equally effective. LAIV has limited ability to spread from person to person, but in general, patients who require protective isolation or are hospitalized should not be exposed to LAIV. Therefore, persons immunized with LAIV should be separated from patients requiring isolation for at least 1 week following immunization.
FAQ 13: is flu vaccination different for adults aged ≥65 years?
Intramuscularly administered Fluzone High-Dose (Sanofi Pasteur) contains 4 times as much antigen as the standard inactivated flu shot and was shown in 1 study to improve on traditionally poor antibody response to standard flu shots in adults ≥65 years and to offer superior protection against influenza.16 New flu vaccines with adjuvants have been recently licensed; ACIP recommendations for elderly or elderly transplant recipients are not yet available.
Meningococcus
N meningitidis causes <1000 US cases per year of meningococcal disease, but the overall case fatality rate exceeds 10%, and another 15% suffer permanent complications including neurological impairment, deafness, and limb loss. Several meningococcal vaccines require consideration. Conjugate (MCV4) vaccines or the traditional polysaccharide vaccine (MPSV4) can provide quadrivalent protection against meningocococcal serogroups A, C, W, and Y (C, W, Y account for ∼70% of US cases). Recently FDA-approved meningococcal serogroup B (Men-B) vaccines now extend protection to serogroup B (up to 33% of US cases). Men-B vaccines are only licensed for individuals 10 to 25 years of age and, until recently, were not commonly used outside of outbreak situations. Vaccination strategies first consider that meningococcal disease is most frequent among infants, preteen/adolescents who are the primary focus group for vaccination, and those over 65 years old among whom 60% of cases are attributable to serogroup Y. Beyond these age groups, other individuals are considered to be at higher risk.
FAQ 14: who should receive which meningococcal vaccine?
We advise that all BMT recipients older than 9 months of age and ≥6 months posttransplant receive 2 doses of a T-dependent conjugated quadrivalent vaccine (MCV4) and not the polysaccharide (MPSV4) vaccine because conjugate vaccines are more immunogenic and stimulate long-lived memory B cells. This is congruent with ACIP policy for anatomic asplenia or functionally immunodeficient such as those with chronic GVHD. Regardless of whether one elects to not routinely vaccinate older BMT recipients who have more rapid IR and no chronic GVHD, environmental risks (military recruits, college freshmen in dormitories, or microbiologists who handle N meningitidis) should be considered.
The ACIP recently advised that a multicomponent Men-B antigen vaccine be given routinely to high-risk individuals ≥10 years old, in addition to MCV4, and may be offered on an individual basis to any adolescent or young adult at a preferred age of 16 to 18 years. This came about because of the availability of safe and immunogenic vaccines and increasing meningococcal B outbreaks.17 We would consider off-label use for >25 year olds with functional or anatomic asplenia (eg, chronic GVHD) or those with workplace risks (see Table 3 and sFAQ 29).
MMR/measles, mumps, rubella, varicella
Measles is a highly contagious airborne respiratory disease that caused ∼2.6 million deaths globally per year before widespread vaccination.19 Measles complications include high fever, rash, blindness, life-threatening diarrhea, encephalitis, or pneumonia. Mumps complications can be more serious in adults than children and include viral meningitis, orchitis, oophoritis, mastitis, and deafness. Rubella complications include polyarthralgia, encephalitis, thrombocytopenia, and potentially devastating congenital rubella syndrome.
FAQ 15: when and how should MMR vaccination be given after BMT?
It is considered safe to give live attenuated MMR when recipients are ≥2 years out from BMT,20 ≥1 year off all systemic IST, and ≥8 months out from any prior IVIG dose (the “2-1-8” mnemonic). Relaxation of this rule to some extent is considered when community outbreaks occur. The efficacy of 2 doses of MMR given 1 month apart in immunocompetent children is ∼97% for measles, ∼88% for mumps, and >90% for rubella. Unvaccinated adults need only 1 dose of MMR. Antibody titers are unnecessary before or after vaccination.
Pneumococcus
Invasive pneumococcal disease (IPD), caused by Streptococcus pneumonia, may result in frequent hospitalizations for fatal pneumonia, bacteremia, or meningitis and is one of the most common vaccine-preventable infections recorded after BMT. At highest risk are adults aged ≥65, the immunocompromised, or those with chronic health conditions.
FAQ 16: how do the 2 available pneumococcal vaccines types differ?
PPSV23 (Pneumovax) is the original polysaccharide capsular vaccine offering protection against 23 serotypes of pneumococcus known to cause disease in humans (Table 4). Twelve of these serotypes plus the 6A serotype not contained in PPSV23 account for >50% of IPD in children and form the basis of the more effective protein conjugated PCV13 vaccine (Prevnar) that was developed originally for young children. Among immunocompromised children, another 25% of IPD cases involved additional serotypes included in PPSV23. Post-2000 herd immunity that followed introduction of the earlier PCV7 vaccine in children also led to a reduction in IPD among older children and adults.
PCV13, a T-cell-dependent vaccine, is more immunogenic than PPSV23 because it triggers memory response that leads to more durable protection than PPSV23, which confers only 3 to 5 years of protection. It is worth remembering that PPSV23 is particularly ineffective in young children age <2 years.
FAQ 17: what pneumococcal vaccination schedule is ideal after BMT?
Unless a patient is severely immunocompromised, we typically begin PCV13 vaccination at ≥6 months, with 3 doses, 1 to 2 months apart (Figure 2).21,22 One dose of PPSV23 is then given 6 to 12 months (minimum 8 weeks) after the last PCV13. The goal is broader coverage to all 23 pneumococcal serotypes (see sFAQs 31, 32). Starting at month 3 vs 9 with PCV7 was equally effective, but pneumococcal titers declined faster for the month 3 cohort; therefore, a fourth dose is advised.21 Priming for PPSV23 vaccination was inferior if vaccination began at month 3. PCV13 responses were inferior for older recipients, donors other than matched siblings, and IgG <400 mg/dL.22 Cord blood or haplorelated grafts were not addressed, and extensive chronic GVHD occurred infrequently in both studies.
FAQ 18: are there exceptions or modifications to the pneumococcal vaccination schedule in FAQ 17?
Yes. When a BMT recipient remains heavily immunocompromised, a fourth dose of PCV13 is given rather than PPSV23 because PCV13 should induce better T-cell collaboration and anamnestic response via generation of memory B cells (see FAQ 5).
Elderly BMT recipients also need PPSV23 booster immunization because of their increased vulnerability to IPD. The baseline assumption is that these individuals earlier completed 3 post-BMT doses of PCV13 and 1 dose of PPSV23, or 4 doses of PCV13. ACIP recommends 1 dose of PCV13 for all adults ≥65 who have not yet received this, followed by a booster PPSV23 6 to 12 months later or a repeat dose of PPSV23 5 years after the last dose of PPSV23. Injection site reactogenicity to PPSV23 is less of an issue if boosting is done infrequently at a time when antibody levels have waned.
Varicella and zoster
FAQ 19: who should be offered varicella vaccine and when is it safe to do so?
Vaccination with Varivax (Merck & Co. Inc.) to prevent chicken pox is only recommended for VZV-seronegative recipients without a history of chickenpox or varicella vaccination because BMT does not eradicate latent VZV in the sensory nerve ganglia of previously infected individuals (ie, those with a history of chicken pox) or previously vaccinated individuals. Latent VZV is thought to provide ongoing antigen exposure that obviates the need for revaccination with standard Varivax. However, a history of varicella does not later preclude the need for high-titer Zostavax (Merck) in ≥60 year olds for the prevention of shingles (see sFAQs 36-40).
Timing of varicella vaccination can be remembered as for MMR by the “2-1-8” rule (see FAQ 15). A second dose of varicella vaccine is needed >1 month after the first.
Addressing vaccine-hesitant patients/parents
FAQ 20: how to address vaccine hesitancy/resistance in patients/parents?
There is no single best way to tackle this problem. Prospective pediatric studies led Opel et al to advise being presumptive (eg, “It is time to do shots”) rather than being participatory (eg, “What do you want to do about shots?”).23 Participatory approaches resulted in more parents verbalizing initial resistance to 1 or more vaccines, even after controlling for vaccine-hesitancy status. In contrast, when the provider just states which vaccines a child is due to receive, most parents have no issues with vaccines. It is fine to presume that the parent you are seeing will be one of the majority. Middle ground might include being presumptive while offering parents an opportunity to express their own preference (eg, “Today, Johnny gets his DTaP, Prevnar, and Hib. Sound OK?”). If a participatory approach is preferred, commit to pursuing any refusals, concerns, or questions knowing that approximately half of the initially vaccine-resistant parents and a quarter of vaccine-hesitant parents will change their mind (see sFAQ 4-6).
Although there is no evidence for vaccination with anything but the recommended schedule, if we have thoroughly explored the reasons for delaying or refusing vaccination and have been unable to convince them otherwise, we would argue that alternative schedules that at least partially immunize the child (patient) are probably better than the alternative of requesting that the parent (patient) seek care elsewhere.
The online version of this article contains a data supplement.
Authorship
Contribution: P.A.C. conceived the FAQ format and wrote the manuscript; J.A.E. critically read and edited the manuscript and provided additional in-depth expertise on technical and clinical aspects of vaccines.
Conflict-of-interest disclosure: J.A.E. has been a consultant for Pfizer and Gilead and receives research support from Roche, Pfizer, GSK, Chimerix, and Novavax. P.A.C. declares no competing financial interests.
Correspondence: Paul A. Carpenter, Fred Hutchinson Cancer Research Center, Mailstop D5-290, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail: pcarpent@fredhutch.org.