Key Points
Elotuzumab, an immunostimulatory antibody, prolongs PFS with no added clinical toxicity when combined with Bd vs Bd alone in RRMM.
Based on results from this phase 2 study, further investigation of elotuzumab with a proteasome inhibitor in RRMM is warranted.
Abstract
In this proof-of-concept, open-label, phase 2 study, patients with relapsed/refractory multiple myeloma (RRMM) received elotuzumab with bortezomib and dexamethasone (EBd) or bortezomib and dexamethasone (Bd) until disease progression/unacceptable toxicity. Primary endpoint was progression-free survival (PFS); secondary/exploratory endpoints included overall response rate (ORR) and overall survival (OS). Two-sided 0.30 significance level was specified (80% power, 103 events) to detect hazard ratio (HR) of 0.69. Efficacy and safety analyses were performed on all randomized patients and all treated patients, respectively. Of 152 randomized patients (77 EBd, 75 Bd), 150 were treated (75 EBd, 75 Bd). PFS was greater with EBd vs Bd (HR, 0.72; 70% confidence interval [CI], 0.59-0.88; stratified log-rank P = .09); median PFS was longer with EBd (9.7 months) vs Bd (6.9 months). In an updated analysis, EBd-treated patients homozygous for the high-affinity FcγRIIIa allele had median PFS of 22.3 months vs 9.8 months in EBd-treated patients homozygous for the low-affinity allele. ORR was 66% (EBd) vs 63% (Bd). Very good partial response or better occurred in 36% of patients (EBd) vs 27% (Bd). Early OS results, based on 40 deaths, revealed an HR of 0.61 (70% CI, 0.43-0.85). To date, 60 deaths have occurred (28 EBd, 32 Bd). No additional clinically significant adverse events occurred with EBd vs Bd. Grade 1/2 infusion reaction rate was low (5% EBd) and mitigated with premedication. In patients with RRMM, elotuzumab, an immunostimulatory antibody, appears to provide clinical benefit without added clinically significant toxicity when combined with Bd vs Bd alone. Registered to ClinicalTrials.gov as NCT01478048.
Introduction
Multiple myeloma (MM) is a malignant disease of monoclonal plasma cells, with a 5-year survival rate below 50%.1 Owing to the increasing aging population, the incidence of MM in the United States is projected to increase by 57% from 2010 to 2030.2 Current choices of care for the treatment of both newly diagnosed and relapsed or refractory multiple myeloma (RRMM) include bortezomib in combination with dexamethasone (Bd).3 However, the disease remains largely incurable, and patients inevitably relapse following therapy or become drug refractory. Despite recent progress in drug development, new treatment modalities are still needed to improve both short-term and long-term treatment outcomes and to overcome drug resistance seen with currently available pharmacotherapies.
Immuno-oncology therapies have potential for long-term survival benefits.4,5 Elotuzumab is a humanized immunoglobulin G1 (IgG1) immunostimulatory monoclonal antibody targeted against Signaling Lymphocytic Activation Molecule Family Member 7 receptor (SLAMF7, formerly CS1 [cell-surface glycoprotein CD2 subset 1]), a glycoprotein expressed on natural killer cells and highly expressed on more than 95% of myeloma cells but not on normal tissues.6 Elotuzumab works in part via a dual mechanism of action, both by directly activating natural killer cells and by binding to FcγRIIIa (CD16a) receptors on natural killer cells, resulting in antibody-dependent cell-mediated cytotoxicity (ADCC) and targeted myeloma cell death.7,8
Elotuzumab showed enhanced activity when combined with bortezomib in a preclinical myeloma model.9 In a phase 1 dose-escalation safety study, IV elotuzumab plus Bd (EBd) was well tolerated in patients with RRMM, with an overall response rate (ORR) of 48% and median time to progression of 9.5 months, which suggests improved activity compared with bortezomib alone.10 We therefore hypothesized that the addition of elotuzumab to Bd would increase progression-free survival (PFS) relative to Bd alone in patients with RRMM.
The objective of this open-label, randomized, phase 2 study was to investigate the efficacy and safety of EBd compared with Bd alone in patients with RRMM.
Patients and methods
Trial design
This was a multicenter, proof-of-concept, signal-generating, open-label, randomized phase 2 study (ClinicalTrials.gov identifier: NCT01478048). The study design and treatment regimens are shown in supplemental Figure 1, available on the Blood Web site. Patients were randomized to EBd or Bd in a 1:1 ratio stratified according to prior proteasome inhibitor (PI) therapy (yes or no), presence of at least 1 FcγRIIIa V allele, and number of prior lines of therapy (1 vs 2 or 3). Treatment was administered in 21-day cycles for cycles 1 to 8 and then in 28-day cycles until disease progression or unacceptable toxicity. Elotuzumab (10 mg/kg IV) was administered weekly for cycles 1 and 2, on days 1 and 11 for cycles 3 to 8, and then on days 1 and 15 thereafter. Bortezomib (1.3 mg/m2 IV or subcutaneously) was administered on days 1, 4, 8, and 11 for cycles 1 to 8 and then on days 1, 8, and 15 thereafter. Dexamethasone 20 mg was administered orally on non-elotuzumab dosing days, and as 8 mg orally plus 8 mg IV on elotuzumab dosing days. Gradual escalation of the elotuzumab infusion rate to a maximum of 5 mL/minute was permitted for patients who had at least 4 consecutive cycles of elotuzumab with no grade 2 or higher infusion reactions (IRs). A premedication regimen was administered prior to each elotuzumab infusion.
Ethics
This study was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from all patients. The protocol, amendments, and patient-informed consent received approval by the appropriate institutional review boards and independent ethics committees prior to initiation of the study.
Patients
Patients were eligible if they were aged 18 years or older with a confirmed diagnosis of MM and had documented progression after 1 to 3 prior lines of therapy. Other inclusion criteria were Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2, confirmed disease progression per International Myeloma Working Group (IMWG) criteria during or after the most recent therapy, and measurable disease according to IMWG criteria.11 Prior PI therapy was allowed if patients did not discontinue a PI as a result of intolerance or grade 3 or higher toxicity, had previously achieved a partial response (PR) or better on a previous PI therapy, and were not refractory to any PI (defined as progression during treatment or within 60 days after the last dose).
Selected key exclusion criteria were the following: clinically significant cardiac disease, prior or concurrent malignancy, neuropathy with pain, or any grade 2 or higher neuropathy.
Efficacy endpoints and assessments
The primary efficacy endpoint was PFS. Secondary and exploratory efficacy endpoints included ORR, time to response, duration of response (DOR), and overall survival (OS).
Under the primary definition (intent to treat), PFS was the time from randomization to the date of the first documented tumor progression or death due to any cause. Clinical deterioration that did not meet the IMWG criteria for progression was not considered progression. The following censoring rules were applied for the primary definition of PFS: a patient who neither progressed nor died was censored on the date of the last adequate tumor assessment requiring both serum and urine M-protein assessments; a patient who did not have any post-baseline tumor assessments and who did not die was censored on the date of randomization.
The secondary definition of PFS was the time from randomization to the date of the first documented tumor progression or death due to any cause, provided that progression or death did not occur after start of subsequent systemic therapy, or more than 10 weeks after the last adequate tumor assessment. Clinical deterioration that did not meet the IMWG criteria for progression was not considered progression. The following censoring rules were considered for the secondary definition of PFS: patients who received subsequent systemic antimyeloma therapy prior to documented progression were censored at the date of the last adequate tumor assessment prior to, or on (ie, if the dates coincide), the initiation of the new therapy. Patients who had an event (documented progression or death) more than 10 weeks (2 assessment visits) after the previous adequate tumor assessment prior to event were censored on that previous adequate assessment date. Patients who neither received subsequent therapy prior to progression nor had a progression event (including death) were censored at their last adequate tumor assessment. In addition, patients who did not have any post-baseline tumor assessments and who did not die within 10 weeks of randomization were censored on the date of randomization. In all cases, if there were no adequate assessments for censoring post-baseline, then the patient was censored on the randomization date.
Tumor assessments for response and progression were conducted on day 1 of every cycle ±7 days until progression and were evaluated using the modified IMWG criteria.11 Objective response for ORR included stringent complete response (CR), CR, very good partial response (VGPR), or PR, per IMWG criteria.11
Safety assessments
Safety evaluations included assessments of serious adverse events (SAEs) and nonserious adverse events (AEs) graded by the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0,12 clinical laboratory tests, and physical examination with assessment of ECOG PS.
Statistical analysis
Approximately 150 patients were planned for randomization. In this proof-of-concept, signal-generating study with an approved agent as the control arm and a time-to-progression primary endpoint, a 2-sided 0.30 significance level was specified to test for differences in PFS between treatment arms (P value ≤ .3 was considered statistically significant for the primary analysis). The study had 80% power to detect a hazard ratio (HR) of 0.69 with 103 events. The primary comparison of PFS was performed using a log-rank test stratified by the stratification factors used during randomization and the primary definition of PFS. The PFS HR, along with a 2-sided 70% confidence interval (CI) corresponding to the type I error for the α (0.3), were calculated using a Cox proportional hazards model, stratified by the factors used during randomization, with treatment as the sole covariate. The nominal 95% CI was provided for reference only. PFS was also analyzed using the secondary definition of PFS. A sensitivity analysis of PFS (primary definition) was performed using an unstratified multivariate Cox regression model in order to estimate the treatment effect after adjustment for possible imbalances in prespecified potential prognostic factors. This model consisted of the following baseline covariates, in addition to the treatment arm as randomized: prior PI use, at least 1 FcγRIIIa V allele, number of prior lines of therapy, age, ECOG PS, prior stem cell transplantation, best response to last therapy, creatinine clearance, and lactate dehydrogenase.
Efficacy analyses were performed on all randomized patients. All patients who received at least 1 dose of study treatment were analyzed for safety. The OS HR, along with a 2-sided 95% CI, were calculated using a Cox proportional hazards model, stratified by the stratification factors used during randomization, with treatment as the sole covariate. The 70% CI was provided for reference. Unless specified otherwise, 95% CI was reported for all other analyses.
All authors had full access to all the data in the study. The study sponsor, Bristol-Myers Squibb, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Results
Patient disposition and baseline characteristics
A total of 152 patients recruited between January 2012 and April 2013 were randomized to EBd (n = 77) or Bd (n = 75; supplemental Figure 2), and 150 patients were treated (EBd, 75; Bd, 75). Demographics were similar across both treatment groups (Table 1). The mean age was 65 years; 19% in the overall population were aged 75 years or older. Approximately half of the patients (51%) had received prior bortezomib therapy. The cutoff date for the primary analysis of PFS and early OS analysis was September 12, 2014. At the cutoff date for the current, updated data analysis (August 10, 2015), 8% of patients treated with EBd vs 1% of patients treated with Bd remained on therapy (supplemental Table 1). The median number of treatment cycles was 12 with EBd and 7 with Bd. Discontinuation in the overall population was mostly due to disease progression (57%).
Baseline demographics
| Characteristic . | EBd (n = 77) . | Bd (n = 75) . | Total (n = 152) . |
|---|---|---|---|
| Age | |||
| Mean (range), y | 65 (25-82) | 65 (30-85) | 65 (25-85) |
| Age group, n (%) | |||
| <65 y | 34 (44) | 33 (44) | 67 (44) |
| ≥65 y | 43 (56) | 42 (56) | 85 (56) |
| ≥75 y | 15 (19) | 14 (19) | 29 (19) |
| Male sex, n (%) | 42 (55) | 37 (49) | 79 (52) |
| Race, n (%) | |||
| White | 68 (88) | 65 (87) | 133 (88) |
| Black/African American | 4 (5) | 7 (9) | 11 (7) |
| ECOG PS, n (%) | |||
| 0 | 38 (49) | 46 (61) | 84 (55) |
| 1 | 35 (46) | 23 (31) | 58 (38) |
| 2 | 2 (3) | 6 (8) | 8 (5) |
| Not reported | 2 (3) | 0 | 2 (1) |
| Prior lines of therapy, n (%) | |||
| 1 | 50 (65) | 51 (68) | 101 (66) |
| 2 or 3 | 27 (35) | 24 (32) | 51 (34) |
| Months since diagnosis | |||
| Median (range) | 45 (9-296) | 44 (8-285) | 45 (8-296) |
| Prior PI use per IVRS, n (%) | |||
| Yes | 38 (49) | 37 (49) | 75 (49) |
| No | 39 (51) | 38 (51) | 77 (51) |
| ISS stage, n (%) | |||
| I | 26 (34) | 19 (25) | 45 (30) |
| II | 23 (30) | 20 (27) | 43 (28) |
| III | 11 (14) | 16 (21) | 27 (18) |
| Not reported | 17 (22) | 20 (27) | 37 (24) |
| Risk category, n (%) | |||
| High* | 0 | 5 (7) | 5 (3) |
| Low† | 0 | 3 (4) | 3 (2) |
| Standard‡ | 36 (47) | 25 (33) | 61 (40) |
| Not evaluable | 41 (53) | 42 (56) | 83 (55) |
| Characteristic . | EBd (n = 77) . | Bd (n = 75) . | Total (n = 152) . |
|---|---|---|---|
| Age | |||
| Mean (range), y | 65 (25-82) | 65 (30-85) | 65 (25-85) |
| Age group, n (%) | |||
| <65 y | 34 (44) | 33 (44) | 67 (44) |
| ≥65 y | 43 (56) | 42 (56) | 85 (56) |
| ≥75 y | 15 (19) | 14 (19) | 29 (19) |
| Male sex, n (%) | 42 (55) | 37 (49) | 79 (52) |
| Race, n (%) | |||
| White | 68 (88) | 65 (87) | 133 (88) |
| Black/African American | 4 (5) | 7 (9) | 11 (7) |
| ECOG PS, n (%) | |||
| 0 | 38 (49) | 46 (61) | 84 (55) |
| 1 | 35 (46) | 23 (31) | 58 (38) |
| 2 | 2 (3) | 6 (8) | 8 (5) |
| Not reported | 2 (3) | 0 | 2 (1) |
| Prior lines of therapy, n (%) | |||
| 1 | 50 (65) | 51 (68) | 101 (66) |
| 2 or 3 | 27 (35) | 24 (32) | 51 (34) |
| Months since diagnosis | |||
| Median (range) | 45 (9-296) | 44 (8-285) | 45 (8-296) |
| Prior PI use per IVRS, n (%) | |||
| Yes | 38 (49) | 37 (49) | 75 (49) |
| No | 39 (51) | 38 (51) | 77 (51) |
| ISS stage, n (%) | |||
| I | 26 (34) | 19 (25) | 45 (30) |
| II | 23 (30) | 20 (27) | 43 (28) |
| III | 11 (14) | 16 (21) | 27 (18) |
| Not reported | 17 (22) | 20 (27) | 37 (24) |
| Risk category, n (%) | |||
| High* | 0 | 5 (7) | 5 (3) |
| Low† | 0 | 3 (4) | 3 (2) |
| Standard‡ | 36 (47) | 25 (33) | 61 (40) |
| Not evaluable | 41 (53) | 42 (56) | 83 (55) |
IVRS, interactive voice response system.
High risk: ISS stage II or III and t(4;14) or del(17p) abnormality.
Low risk: ISS stage I or II and absence of t(4;14), del(17p), and 1q21 abnormalities and age <55 y.
Standard risk: patients not meeting the definition of high or low risk.
Dose intensity
The planned dose intensity of each agent in the 2 groups is shown in supplemental Table 2. Most patients (73%) received the full dose (≥90%) of elotuzumab in the EBd arm. Dose intensities for Bd (range <60% to ≥90% for each) were similar in both treatment arms and were not impacted by the addition of elotuzumab.
Efficacy
Primary and secondary endpoints.
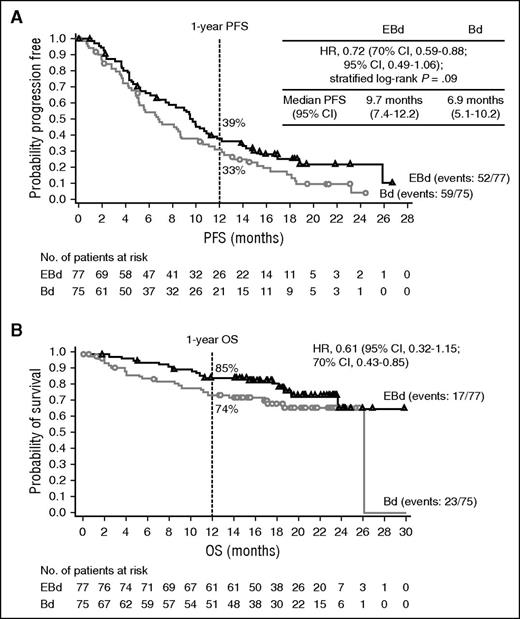
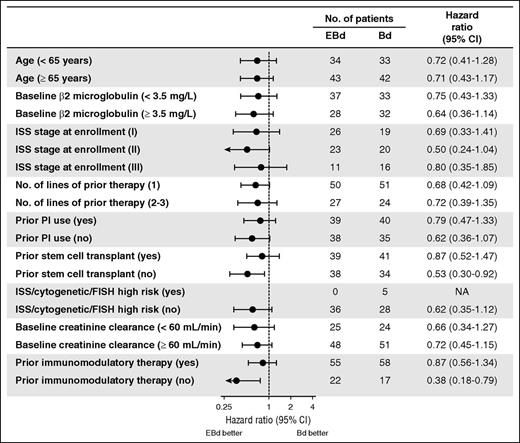
In the primary analysis, a total of 111 of 152 patients had a PFS event. For the 41 patients who did not have a PFS event (25 EBd; 16 Bd), the median follow-up time since randomization was 15.9 months for the EBd group and 11.7 months for the Bd group. The study met its primary endpoint of PFS, with an HR of 0.72 (70% CI, 0.59-0.88; stratified log-rank P = .09), indicating a 28% reduction in the risk of progression or death with EBd compared with Bd. Median PFS was 9.7 months with EBd vs 6.9 months with Bd (Figure 1A). The 1-year PFS rate was 39% (95% CI, 28%-50%) with EBd vs 33% (95% CI, 22%-44%) with Bd. In addition, analysis using the secondary definition of PFS yielded an HR of 0.66 (70% CI, 0.52-0.83; stratified log-rank P = .06) and a median PFS of 9.7 months with EBd vs 6.6 months with Bd (supplemental Figure 3). A trend toward longer PFS with EBd was observed across key subgroups, including in patients aged 65 years or older and those who had received a prior PI or immunomodulatory therapy (Figure 2). A sensitivity analysis of PFS (primary definition), adjusting for possible imbalances in prespecified prognostic factors (detailed in “Statistical analysis”), yielded an estimated PFS HR of 0.53 (70% CI, 0.42-0.66; P = .0039).
Survival rates. (A) PFS (primary definition) and (B) OS. Data cutoff: September 12, 2014.
Survival rates. (A) PFS (primary definition) and (B) OS. Data cutoff: September 12, 2014.
PFS (primary definition): subgroup analysis. FISH, fluorescence in situ hybridization; ISS, International Staging System; NA, not available; PI, proteasome inhibitor. Data cutoff: September 12, 2014.
PFS (primary definition): subgroup analysis. FISH, fluorescence in situ hybridization; ISS, International Staging System; NA, not available; PI, proteasome inhibitor. Data cutoff: September 12, 2014.
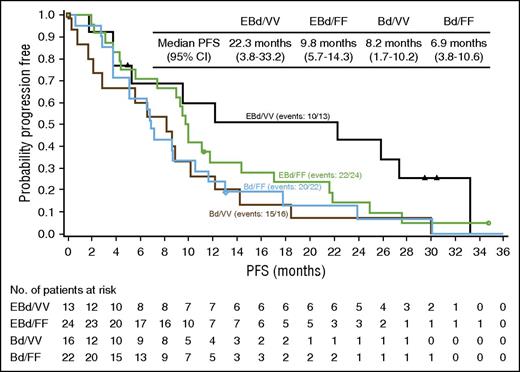
In the updated analysis, the 2-year PFS rate was 18% (95% CI, 10%-28%) with EBd vs 11% (95% CI, 5%-20%) with Bd (supplemental Figure 4A). Subgroup analysis was consistent between 1 and 2 years (supplemental Figure 5). Patients in the EBd group who were homozygous for the high-affinity FcγRIIIa V (VV) allele (13 patients) had a median PFS of 22.3 months compared with 9.8 months in patients in the EBd group homozygous for the low-affinity FcγRIIIa F (FF) allele (24 patients) and a sizable improvement compared with patients in the Bd group homozygous for the V allele (8.2 months; Figure 3). However, even patients in the EBd group harboring the low-affinity FcγRIIIa genotype appeared to have higher PFS compared with those in the Bd group. Patients in the Bd group showed similar PFS regardless of FcγRIIIa genotype.
PFS (primary definition) in FcγRIIIa high-affinity (VV) and low-affinity (FF) subgroups. Data are based on all randomized patients with FcγRIIIa genotypes being homozygous VV or FF. Data cutoff: August 10, 2015.
PFS (primary definition) in FcγRIIIa high-affinity (VV) and low-affinity (FF) subgroups. Data are based on all randomized patients with FcγRIIIa genotypes being homozygous VV or FF. Data cutoff: August 10, 2015.
In the updated analysis, ORR (PR or better) was 66% (95% CI, 55%-77%) with EBd vs 63% (95% CI, 51%-74%) with Bd (Table 2). Response rates of VGPR or better occurred in 36% (28/77) of patients with EBd vs 27% (20/75) of patients with Bd (Table 2).
Overall response rate and best overall response
| Treatment response . | EBd (n = 77) . | Bd (n = 75) . |
|---|---|---|
| Overall response rate, n (%)* | 51 (66) | 47 (63) |
| 95% CI | 55-77 | 51-74 |
| Best overall response, n (%) | ||
| Stringent CR | 0 | 1 (1) |
| CR | 3 (4) | 2 (3) |
| Very good partial response | 25 (33) | 17 (23) |
| Partial response | 23 (30) | 27 (36) |
| Minimal response | 4 (5) | 5 (7) |
| Stable disease | 13 (17) | 14 (19) |
| Progressive disease | 4 (5) | 4 (5) |
| Not evaluable | 5 (7) | 5 (7) |
| Treatment response . | EBd (n = 77) . | Bd (n = 75) . |
|---|---|---|
| Overall response rate, n (%)* | 51 (66) | 47 (63) |
| 95% CI | 55-77 | 51-74 |
| Best overall response, n (%) | ||
| Stringent CR | 0 | 1 (1) |
| CR | 3 (4) | 2 (3) |
| Very good partial response | 25 (33) | 17 (23) |
| Partial response | 23 (30) | 27 (36) |
| Minimal response | 4 (5) | 5 (7) |
| Stable disease | 13 (17) | 14 (19) |
| Progressive disease | 4 (5) | 4 (5) |
| Not evaluable | 5 (7) | 5 (7) |
Data cutoff: August 10, 2015.
Overall response rate was defined as partial response or better, according to the modified IMWG criteria.
Exploratory endpoints.
Median time to response was 1.4 months (6.1 weeks) in the EBd group and 1.5 months (6.5 weeks) in the Bd group. DOR was 11.4 months (95% CI, 8.5-14.1 months) in the EBd group and 9.3 months (95% CI, 5.6-11.7 months) in the Bd group.
Although survival data are immature, early OS results based on 40 deaths (17 in EBd group and 23 in Bd) revealed a 1-year rate of 85% (95% CI, 75%- 92%) in the EBd group vs 74% (95% CI, 62%-83%) in the Bd group (Figure 1B), and an HR of 0.61 (95% CI, 0.32-1.15; 70% CI, 0.43-0.85). In an updated analysis based on 60 deaths (28 in EBd group and 32 in Bd), the 2-year OS rate was 73% (95% CI, 61%-82%) with EBd vs 66% (95% CI, 54%-76%) with Bd (supplemental Figure 4B). Follow-up for OS continues.
Natural killer cell dynamics.
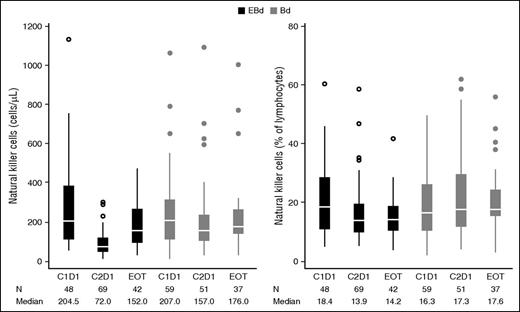
There was a general decline in total circulating natural killer cell count after initial doses of therapy (Figure 4), particularly observed on day 1 of cycle 2 for both treatment groups. However, the decline appeared to be more pronounced in the EBd group. This effect was transient as circulating natural killer cell numbers recovered to near-baseline levels by the end of therapy (discontinuation of elotuzumab).
Peripheral blood natural killer cell counts/percentage (all treated patients). Baseline natural killer cell values are reported on cycle 1 day 1 (C1D1). C2D1, cycle 2 day 1; EOT, end of treatment (discontinuation of elotuzumab). Whiskers, confidence intervals; middle line in box, median; upper and lower limit of box, range; circles, outliers.
Peripheral blood natural killer cell counts/percentage (all treated patients). Baseline natural killer cell values are reported on cycle 1 day 1 (C1D1). C2D1, cycle 2 day 1; EOT, end of treatment (discontinuation of elotuzumab). Whiskers, confidence intervals; middle line in box, median; upper and lower limit of box, range; circles, outliers.
Safety
In the updated analysis, AEs were reported in 75 patients (100%) treated with EBd and 72 patients (96%) treated with Bd. There appeared to be minimal differences in AEs between arms. AEs occurring in at least 25% of patients are shown in Table 3. Grade 3 to 4 AEs were reported in 53 patients (71%) with EBd vs 45 patients (60%) with Bd. The most common grade 3 or higher AEs were infections (EBd, 16 [21%]; Bd, 10 [13%]) and thrombocytopenia (EBd, 7 [9%]; Bd, 13 [17%]). AEs leading to study drug discontinuation occurred in 24 patients (32%) in the EBd group and 29 patients (39%) in the Bd group.
Adverse events in at least 25% of patients
| . | EBd (n = 75) . | Bd (n = 75) . | ||
|---|---|---|---|---|
| Events* . | Any grade† . | Grade 3-4 . | Any grade† . | Grade 3-4 . |
| All AEs | 75 (100) | 53 (71) | 72 (96) | 45 (60) |
| Infections | 50 (67) | 16 (21) | 40 (53) | 10 (13) |
| Diarrhea | 33 (44) | 6 (8) | 25 (33) | 3 (4) |
| Constipation | 30 (40) | 1 (1) | 22 (29) | 0 |
| Cough | 33 (44) | 1 (1) | 18 (24) | 0 |
| Anemia | 28 (37) | 5 (7) | 22 (29) | 5 (7) |
| Peripheral neuropathy | 27 (36) | 7 (9) | 27 (36) | 9 (12) |
| Pyrexia | 28 (37) | 0 | 21 (28) | 3 (4) |
| Peripheral edema | 22 (29) | 3 (4) | 18 (24) | 0 |
| Insomnia | 22 (29) | 1 (1) | 14 (19) | 1 (1) |
| Asthenia | 21 (28) | 3 (4) | 22 (29) | 2 (3) |
| Fatigue | 22 (29) | 3 (4) | 19 (25) | 1 (1) |
| Paresthesia | 20 (27) | 0 | 14 (19) | 4 (5) |
| Nausea | 20 (27) | 1 (1) | 16 (21) | 1 (1) |
| Thrombocytopenia | 12 (16) | 7 (9) | 20 (27) | 13 (17) |
| . | EBd (n = 75) . | Bd (n = 75) . | ||
|---|---|---|---|---|
| Events* . | Any grade† . | Grade 3-4 . | Any grade† . | Grade 3-4 . |
| All AEs | 75 (100) | 53 (71) | 72 (96) | 45 (60) |
| Infections | 50 (67) | 16 (21) | 40 (53) | 10 (13) |
| Diarrhea | 33 (44) | 6 (8) | 25 (33) | 3 (4) |
| Constipation | 30 (40) | 1 (1) | 22 (29) | 0 |
| Cough | 33 (44) | 1 (1) | 18 (24) | 0 |
| Anemia | 28 (37) | 5 (7) | 22 (29) | 5 (7) |
| Peripheral neuropathy | 27 (36) | 7 (9) | 27 (36) | 9 (12) |
| Pyrexia | 28 (37) | 0 | 21 (28) | 3 (4) |
| Peripheral edema | 22 (29) | 3 (4) | 18 (24) | 0 |
| Insomnia | 22 (29) | 1 (1) | 14 (19) | 1 (1) |
| Asthenia | 21 (28) | 3 (4) | 22 (29) | 2 (3) |
| Fatigue | 22 (29) | 3 (4) | 19 (25) | 1 (1) |
| Paresthesia | 20 (27) | 0 | 14 (19) | 4 (5) |
| Nausea | 20 (27) | 1 (1) | 16 (21) | 1 (1) |
| Thrombocytopenia | 12 (16) | 7 (9) | 20 (27) | 13 (17) |
Data are n (%) of patients. Data cutoff: August 10, 2015.
AEs were categorized using the Medical Dictionary for Regulatory Activities and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3).12
Grade 5 AEs occurred in 4 patients in the EBd group and 6 patients in the Bd group.
SAEs were reported in 38 patients (51%) in the EBd group and 31 patients (41%) in the Bd group (supplemental Table 3).
On-study deaths (occurring during therapy or within 60 days of the last dose of study treatment) occurred in 2 patients (3%) in the EBd group and 6 patients (8%) in the Bd group. The primary cause of on-study deaths was disease (2 patients) in the EBd group, and disease (2 patients), cardiovascular disease (2 patients), infection (1 patient), and fatal bleeding (1 patient) in the Bd group.
Infusion reactions
Grade 1 to 2 IRs occurred in 4 patients (5%) in the EBd group (pyrexia in 2 patients, and bone pain, chills, flushing, nausea, and ear pruritus in 1 patient each). The rate of IRs was low, and their severity was mitigated with premedication. There were no grade 3 or higher IRs. A total of 27 patients were administered elotuzumab within 1 hour at the 5-mL/minute infusion rate, and 1 patient reported an IR at this higher rate. There were no discontinuations due to IRs.
Discussion
In this proof-of-concept, signal-generating, open-label, randomized phase 2 study, elotuzumab demonstrated efficacy and tolerability in combination with Bd in patients with RRMM. The study met its primary endpoint; PFS was statistically significantly longer with EBd than with Bd (P = .09), exceeding the prespecified significance level of 2-sided P ≤ .3. Patients treated with EBd had a 28% reduction in the risk of disease progression or death compared with those treated with Bd. Similarly, PFS results seem to favor the addition of elotuzumab to Bd across key subgroups, including elderly patients and those who had received 2 or 3 prior lines of therapy. However, as the numbers of patients in these subgroups were relatively small, these findings should be interpreted with caution.
A critical component of natural killer cell–mediated ADCC is the mechanism whereby the Fc portion of the IgG1 antibody is able to bind to the FcγRIIIa receptor expressed on natural killer cells.6,7 The FcγRIIIa gene has allelic variation that confers the affinity of the FcγRIIIa receptor for IgG1 antibodies.6 As such, FcγRIIIa receptor polymorphisms were examined in this study to elucidate associations between genotype and clinical outcome. Although patient numbers were low and findings should be interpreted with caution, the analysis suggests that patients in the EBd group who were homozygous for the high-affinity FcγRIIIa V allele appeared to show longer PFS compared with patients homozygous for the low-affinity allele. Importantly, regardless of FcγRIIIa genotype, patients in the EBd group appeared to demonstrate longer PFS compared with patients in the Bd group. This observation toward longer PFS with EBd in patients homozygous for the high-affinity FcγRIIIa V allele is consistent with natural killer cell–mediated ADCC as a mechanism of action of elotuzumab6,7,9 and has been observed with other immunotherapeutic molecules including rituximab.13,14 Considering the striking difference in PFS among patients with the high-affinity FcγRIIIa V allele compared with those bearing the low-affinity allele, further analysis of this population is needed.
ORR was comparable in the EBd and Bd arms, with no statistical difference between treatment groups. However, patients in the EBd group appeared to have a higher rate of high-quality responses (VGPR or better), and responses appeared to be more durable with EBd. It should be noted that CRs may be underestimated in this study in the EBd arm owing to the presence of elotuzumab in the serum protein electrophoresis and serum immunofixation electrophoresis assays, which comigrates with serum M-protein.15,16 More patients continued therapy with EBd than with Bd at the time of data cutoff, and the main reason for discontinuation was disease progression. Early OS data appear to indicate survival benefit, but no conclusions can be drawn at this time due to the limited number of events.
Taking into account all limitations of cross-study comparisons, it appears that the PFS, ORR, and DOR results in the experimental and control arms seen in the current study are similar to those reported in other studies that evaluated triple combinations with Bd,17-20 suggesting that the addition of elotuzumab to Bd provides similar benefit than addition of another alternative third agent to Bd. In addition, the results obtained with EBd appear to suggest that addition of elotuzumab to Bd provides similar relative benefits as the addition of elotuzumab to lenalidomide and dexamethasone, as shown by a comparable HR in both studies in a similar patient population in RRMM, with a PFS reduction of 28% and 30%, respectively.21
The dual mechanism of action of elotuzumab exerts both a tumoricidal effect by mediating ADCC and an immunostimulatory effect by directly activating natural killer cells. Observation of the dynamics of natural killer cells and the role of the FcγRIIIa V allele may provide some guidance on how elotuzumab can be most effectively used in combination with other agents, including PIs. At the time this study was initiated, it was unclear whether combining elotuzumab with a PI would be as beneficial as combining with lenalidomide or other immunomodulatory drugs (IMiDs), as the effects of PIs on natural killer cells were unknown. This study appears to provide an important proof of concept that the addition of elotuzumab to a PI may be as beneficial as the addition of elotuzumab to IMiDs.
Three-drug combinations for the treatment of MM are consistently more effective than 2-drug combinations, based on a number of randomized trials, but can be limited by incremental toxicity.22-24 It is important to note that in the present study, the combination of EBd was well tolerated, and no meaningful increase in AEs was observed with the addition of a third agent (elotuzumab) to Bd.
Elotuzumab infusions have been associated with predominantly grade 1 or 2 IRs.10,21,25 The IR rates observed in this study were lower than those reported in a randomized phase 3 study of elotuzumab with lenalidomide and low-dose dexamethasone in patients with RRMM and were mitigated with premedication, likely due to the optimal elotuzumab dosing schedule selected and a gradual escalation of the infusion rate to a maximum of 5 mL/minute.21 In addition, IRs in this study were mainly observed in the early treatment cycles.
Limitations of this proof-of-concept study include the small sample size and large type 1 error rate, the open-label study design with no central laboratory analyses, and PFS and ORR results based on investigator assessment rather than an independent review committee. However, these results are consistent with the previously published single-arm phase 1 study of this combination.10 Follow-up for longer-term outcomes, including survival, will continue to inform the role of the addition of elotuzumab to a PI-based regimen.
Phase 2 studies in oncology are largely single-arm trials using response rate as the primary efficacy endpoint; this phase 2 study, however, implemented a randomized, controlled study design with a challenging “head-to-head” comparison with an efficacious, approved regimen, using a time-to-event assessment, PFS, as the primary efficacy endpoint. Results suggest a benefit of adding elotuzumab to Bd based on the prespecified significance level. Because of study design limitations, it is possible that there is a specific subpopulation of patients that is affecting these positive results; therefore, a longer follow-up and further investigation are needed. Although these results are encouraging, it is important to note that this was not a confirmatory study but rather a proof-of-concept study. Nevertheless, the results from this study provide support for combining elotuzumab with PIs or IMiDs.21 Further phase 3 evaluation of these combinations are warranted.
In conclusion, the results of this proof-of-concept, signal-generating, randomized phase 2 study seem to show that the addition of elotuzumab, a first-in-class immunostimulatory monoclonal antibody with the novel mechanism of action of both direct activation and engagement of natural killer cells to trigger ADCC, to Bd results in a longer PFS compared with Bd alone. In addition, the combination was well tolerated. Further investigation of elotuzumab with a PI, including carfilzomib or ixazomib, is warranted.
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015; the 20th Congress of the European Hematology Association, Vienna, Austria, June 11-14, 2015; and the 57th American Society of Hematology Annual Meeting, Orlando, FL, December 5-8, 2015.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Editorial support in the form of writing the first draft, drafting tables, and collating author comments was provided by Kate Jesien at Caudex, New York, under the direction of the authors.
This work was funded by Bristol-Myers Squibb.
Authorship
Contribution: A.J., B.P., A.K.S., L.C.T., M.L., and A.P. were involved in the conception and design of the study; A.J., M.O., J.D.L.R., L.G., A.B., and R.M. provided study materials or recruited patients; A.J., B.P., J.D.L.R., K.L., A.B., A.M.C., A.K.S., M.L., E.B., and Y.-M.J. collected the data; A.J., M.O., B.P., L.G., K.L., R.M., A.M., A.K.S., L.C.T., M.L., E.B., Y.-M.J., and A.P. analyzed and interpreted the data; E.B. provided administrative support; A.J., M.L., R.M., J.L., A.M., A.K.S., E.B., Y.-M.J., M.R., and A.P. wrote the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: A.J. has received institutional funding for support of clinical trial conduct from Bristol-Myers Squibb and has received personal fees (advisory board, consultancy, speaking, and honoraria) from Bristol-Myers Squibb, Celgene, Millennium, Novartis, Onyx, SkylineDx, Karyopharm, and Sanofi-Aventis. M.O. has received honoraria and travel expenses from Janssen and Bristol-Myers Squibb. B.P. has received honoraria from Celgene. J.D.L.R. has provided expert testimony for Amgen, Takeda, and Janssen. L.G. has served on advisory boards for Bristol-Myers Squibb and Amgen and has received honoraria from Bristol-Myers Squibb. A.B. has received honoraria from Janssen, Amgen, and Mundipharma; has served as a consultant for Amgen, Mundipharma, and Sandoz; has participated in a speakers bureau for GlaxoSmithKline, Amgen, ARIAD Pharmaceuticals, and Italfarmaco; has received research funding from Italfarmaco and Teva; and has received travel expenses from Alexion Pharmaceuticals, Celgene, Amgen, and Binding Site. R.M. has received honoraria and travel expenses from Janssen, Celgene, Mundipharma, and Novartis. J.L. has received research funding from Novartis, Onyx, Celgene, and Millennium. A.M. has participated in a speakers bureau for and received travel or accommodation expenses from Genentech and Millennium. A.K.S. and L.C.T. are employees of and own stocks in AbbVie Biotherapeutics. M.L., E.B., Y.-M.J., and M.R. are employees of and own stocks in Bristol-Myers Squibb. A.P. has received consultancy fees, honoraria, and research funding from Amgen, Novartis, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Takeda, Sanofi-Aventis, and Merck; and participated in a speakers bureau for Bristol-Myers Squibb. K.L. has received grant and/or research support from Hospira, Roche, Teva, Janssen-Cilag, Mundipharma, Novartis, and consultancy fees from Amgen and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Andrzej Jakubowiak, University of Chicago Medical Center, 5841 S Maryland Ave, MC 2115, Chicago, IL 60637; e-mail: ajakubowiak@medicine.bsd.uchicago.edu.