To the editor:
von Willebrand factor (VWF) mediates platelet adhesion and agglutination at sites of vascular injury. VWF is a large multimeric glycoprotein, which circulates in a coiled, non-adhesive conformation. When exposed to high shear stress, VWF unfolds and elongates to highly adhesive multimer strands that adhere on exposed collagen fibers, and by interacting with platelet glycoprotein Ib-α, recruit platelets to the injury site.1,2
Recent in vivo work showed that transition from laminar to turbulent flow, as observed at sites of stenotic vessels, is the most important trigger for adhesive conformational change of VWF.3 Additionally, the ability of VWF to tether platelets is directly proportional to its multimeric size. The latter is regulated by ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type-1 repeats, member 13), a metalloprotease that cleaves VWF multimers within the unfolded A2 domain, thereby reducing VWF multimer size, and by this, decreasing VWF adhesiveness.4
We postulated that transition from laminar to turbulent flow at sites of critical coronary stenosis may cause a local imbalance between VWF and ADAMTS13,5 which would be responsible for the presence of highly adhesive VWF multimers mediating platelet adhesion and agglutination, and ultimately responsible for acute occlusion. The aim of this investigation was to evaluate systemic and intracoronary thrombin generation, VWF, and ADAMTS13 activity in patients with acute coronary syndromes (ACS) with angiographic evidence of acute thrombotic coronary occlusion.
We investigated 27 patients (24 men; median age, 65 years; interquartile range [IQR], 54-74; range, 41-84) undergoing emergency cardiac catheterization for hemodynamically stable ACS presenting with ST-elevation myocardial infarction (STEMI) who gave written informed consent. The culprit lesion was determined angiographically in two orthogonal projections and was defined by the combination of: (1) abrupt (typical) vessel occlusion; and (2) complete absence of (or severely impaired) peripheral coronary flow. Infarct localization was anterior in 9 patients, infero-posterior in 16, and lateral in 2. Maximal creatine kinase value was median 1440 U/L (IQR, 922-2262; range, 750-5800) and the extent of the underlying coronary artery disease assessed by the Syntax score was 17 ± 7. All patients underwent percutaneous coronary intervention/stenting with a median time from chest pain to onset-balloon of 105 minutes (IQR, 62-150; range, 20-1140).
Blood samples were obtained at 3 different sites before stenting: from the femoral vein and at the ostia of the right or left coronary artery through a coronary guiding 6 French catheter, and 1-3 cm distal to the site of occlusion with a conventional 6 French compatible thrombus aspiration catheter advanced through the lesion. In a preliminary phase, we verified that blood sampling by coronary catheter did not alter results for thrombin-generation markers, VWF, and ADAMTS13 compared with peripheral venipuncture. Additionally, care was taken to perform extra slow aspiration to avoid turbulences inside the catheter. Samples were collected into citrate test tubes, snap-frozen immediately after centrifugation, and kept at −80°C until batch analysis. Markers of thrombin generation and VWF antigen (VWF:Ag) were assessed by enzyme-linked immunosorbent assay and VWF activity (VWF:RCo) by a turbidimetric method as published.6 ADAMTS13 activity was measured by the fluorescence resonance energy transfer-VWF73 assay as previously described.7
Our results show that parameters of thrombin generation, ie, prothrombin fragments 1+2 (F1+2), thrombin-antithrombin complexes (TAT), and D-dimers did not differ between peripheral and coronary blood (Table 1). This excludes major pre-analytical artifacts and appears to reflect a modest contribution of thrombin generation to acute coronary occlusion.8
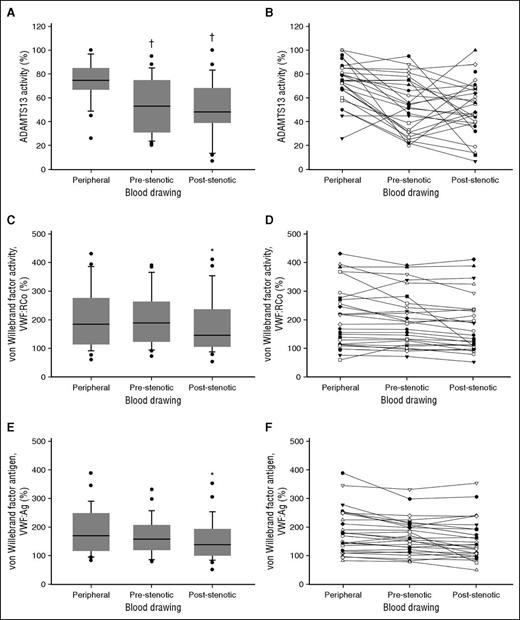
In line with previous reports in ACS patients, we observed markedly increased circulating levels of VWF and a relative reduction in systemic ADAMST13 activity.9-11 Most interestingly, and to our knowledge for the first time, we documented a significantly reduced ADAMTS13 activity in coronary blood compared with systemic levels and a significant gradient of both VWF:RCo and VWF:Ag across the coronary occlusion (Table 1; Figure 1).
VWF, ADAMTS13, and markers of thrombin generation in patients with STEMI. (A-B) ADAMTS13 activity. (C-D) VWF:RCo. (E-F) VWF:Ag. †, statistically significantly different from peripheral blood; *statistically significantly different from peripheral blood and pre-occlusion coronary blood.
VWF, ADAMTS13, and markers of thrombin generation in patients with STEMI. (A-B) ADAMTS13 activity. (C-D) VWF:RCo. (E-F) VWF:Ag. †, statistically significantly different from peripheral blood; *statistically significantly different from peripheral blood and pre-occlusion coronary blood.
These data suggest a pathogenic role for an acquired intracoronary ADAMTS13 deficiency in ACS and indicate that VWF is retained at the site of acute coronary occlusion. In particular, our data support the hypothesis that a decreased ADAMTS13/VWF ratio in the coronary flow favors the presence of highly adhesive VWF multimers that would deposit at the site of a critical stenosis, mediating platelet adhesion and agglutination and, eventually, leading to coronary occlusion. In fact, histologic examination of coronary thrombi aspirated from patients with acute myocardial infarction revealed a prominent co-localization of VWF with platelets.12,13 These observations are in line with animal models showing that ADAMTS13 deficiency exacerbates VWF-dependent thrombus formation on disrupted plaques, thereby impacting also on the resulting infarct size.14,15 A surprising finding was the significantly reduced ADAMTS13 activity in coronary blood compared with systemic levels. Although we do not know the reason for this difference, we speculate that it might be explained by local hemodynamic factors (wide flow variations within cardiac chambers followed by low pressure, high velocity intracoronary flow) and/or changes secondary to the acute coronary occlusion (increased proximal coronary flow resistance and intracoronary shear stress because of vascular bed’s amputation).
In conclusion, our observations support the hypothesis that a significantly reduced ADAMTS13/VWF ratio in the coronary artery flow plays a pathogenic role in ACS and suggest that transition from laminar to turbulent flow at sites of coronary stenosis further enhances VWF activation and deposition. These events would ultimately sustain platelet adhesion and agglutination, and favor coronary occlusion.
The potential therapeutic implication of this concept, to be clinically tested, would be the local infusion of ADAMTS13 to decrease VWF-mediated platelet adhesion and agglutination at sites of critical coronary stenosis.
Authorship
Contribution: G.P. designed the study, performed research, analyzed results, and edited the manuscript; L.B. performed research and edited the manuscript; I.S. and A.A. performed research; T.M. and J.A.K.H. analyzed results and edited the manuscript; and L.A. designed the study, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni Pedrazzini, Fondazione CardioCentro Ticino, Via Tesserete 48, CH-6900 Lugano, Switzerland; e-mail: giovanni.pedrazzini@cardiocentro.org; and Lorenzo Alberio, Service et Laboratoire central d’Hématologie, Centre Hospitalier Universitaire Vaudois, Rue du Bugnon 46, CH-1011 Lausanne, Switzerland; e-mail: lorenzo.alberio@chuv.ch.