In this issue of Blood, Avellino et al characterize an enhancer element within the CCAAT enhancer-binding protein α (C/EBPα) locus that is essential for hematopoietic stem cell (HSC) differentiation and the development of mature myeloid cells.1
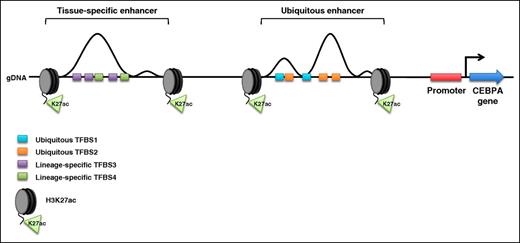
Schematic of the human CEBPA locus. Enhancer elements upstream of the CEBPA promoter are flanked by acetylated lysine 27 on the tail of histone H3 (H3K27ac). The genomic sequence under each enhancer contains consensus transcription factor–binding sites (TFBS). In all tissues that express CEBPA, transcription factors bind their consensus sequences within the ubiquitous enhancer (ubiquitous TFBS1 and TFBS2). Lineage-specific transcription factors bind their consensus sequences within the tissue-specific enhancer only when CEBPA is expressed in the corresponding cell lineage (lineage-specific TFBS3 and TFBS4). gDNA, genomic DNA.
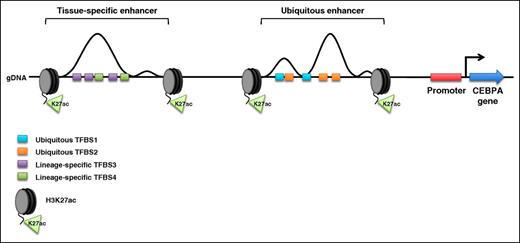
Schematic of the human CEBPA locus. Enhancer elements upstream of the CEBPA promoter are flanked by acetylated lysine 27 on the tail of histone H3 (H3K27ac). The genomic sequence under each enhancer contains consensus transcription factor–binding sites (TFBS). In all tissues that express CEBPA, transcription factors bind their consensus sequences within the ubiquitous enhancer (ubiquitous TFBS1 and TFBS2). Lineage-specific transcription factors bind their consensus sequences within the tissue-specific enhancer only when CEBPA is expressed in the corresponding cell lineage (lineage-specific TFBS3 and TFBS4). gDNA, genomic DNA.
CEBPA is a transcription factor that acts as a master differentiation regulator in many tissues including liver, lung, adipose, and blood.2 Between 10% and 15% of acute myeloid leukemia (AML) subtypes have CEBPA mutations or changes in CEBPA regulation that lead to a block in myeloid differentiation.3-5
Enhancers are regulatory regions of DNA that physically interact with gene promoters to increase transcription initiation rate and frequency. These regions often overlap binding sites for lineage-specific transcription factors that are essential to cell-fate decisions, and often work additively to enhance gene expression. Although the effect of enhancer activity on gene expression is well understood, the significance of a single enhancer on an individual cell’s commitment to a given lineage is more broadly debated. Avellino et al use an elegant series of experiments to investigate lineage-specific use of a single enhancer that is critical for myelopoiesis.
To better understand the effect of CEBPA enhancer elements on lineage commitment, Avellino and colleagues investigated a 170-kb genomic domain that physically contacts the CEBPA promoter. Using the histone H3 lysine 27 acetylation (H3K27ac) epigenetic mark to identify potentially active enhancers, the authors created an epigenetic map that profiled the location of enhancers in 14 human tissues. They identified 2 enhancers of particular interest: the first enhancer is active in all tissue types assayed (they later show that this enhancer positively influences, but is not required for, CEBPA expression in multiple cell types) and the second enhancer (referred to as the +42-kb enhancer) is only active in myeloid cells. Avellino et al subsequently showed that the +42-kb enhancer contains transcription factor–binding sites that are occupied by several critical myeloid transcription factors, including runt-related transcription factor 1 (RUNX1), the Spi-1 proto-oncogene protein product PU.1, and growth factor–independent 1 transcription repressor (GFI2) (see figure).
The experiment that marks Avellino and colleagues’ study as a significant step in elucidating enhancer mechanisms is their investigation of the +42-kb enhancer in vivo. After identifying the homologous domain in the mouse genome, the authors use the clustered regularly-interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) genome-editing system to remove the proposed 1.1-kb–wide myeloid-specific enhancer in the mouse germ line.6 The authors found that removal of this enhancer significantly decreased CEBPA expression specifically in total bone marrow. In addition, unlike previous models that deleted the entire CEBPA gene, removal of the +42-kb enhancer produced viable animals with an HSC self-renewal defect and abnormal expansion of the multipotent progenitor population characteristic of a preleukemic state.2,7,8
The clinical significance of altered CEBPA expression in hematopoiesis has largely been limited to cases involving mutations in the coding region of the single-exon CEBPA gene itself. Although leukemias with CEBPA mutations are often associated with favorable prognoses, an individual’s risk and prognosis can vary by both the type of CEBPα mutation as well as mutations in other genes or chromosomal abnormalities that occur concurrently.3,5 In particular, the RUNX1–RUNX1 translocated to 1 (RUNX1-RUNX1T1, or AML1-ETO) fusion protein that results from the t(8;21) AML translocation constitutes an entire AML subgroup in which CEBPA expression is suppressed due to the mislocalization of the RUNX1 transcription factor.4
The epigenetic regulation of CEBPA by RUNX1 is not itself a novel finding. The mouse enhancer region homologous to the +42-kb enhancer used by Avellino et al was first reported by Guo and colleagues in 2012.9 In that study, 4 occupied RUNX1-binding sites were identified in the enhancer that, when mutated, greatly reduce CEBPA expression in mouse cell lines. However, whereas Guo and colleagues investigated the effect of an inducible in vivo RUNX1 deletion on the HSC compartment, Avellino and colleagues specifically characterize the significance of the CEBPα enhancer on myelopoiesis and bone marrow engraftment competency (see figure).
In summary, Avellino and colleagues have identified a single enhancer required for myeloid priming in HSCs, and their study provides a critical link between enhancer activation, transcription factor binding, lineage commitment, and gene expression. The proof that deletion of a 1.1-kb enhancer can lead to the unchecked expansion of the multipotent progenitor cell population provides new insight into the role of epigenetics and the preleukemic state.
Conflict-of-interest disclosure: The author declares no competing financial interests.