Key Points
Coagulation initiation on epithelia activates the membrane-anchored serine protease matriptase.
Matriptase mediates epithelial proteolysis and signaling by coagulation proteases.
Abstract
The coagulation cascade is designed to sense tissue injury by physical separation of the membrane-anchored cofactor tissue factor (TF) from inactive precursors of coagulation proteases circulating in plasma. Once TF on epithelial and other extravascular cells is exposed to plasma, sequential activation of coagulation proteases coordinates hemostasis and contributes to host defense and tissue repair. Membrane-anchored serine proteases (MASPs) play critical roles in the development and homeostasis of epithelial barrier tissues; how MASPs are activated in mature epithelia is unknown. We here report that proteases of the extrinsic pathway of blood coagulation transactivate the MASP matriptase, thus connecting coagulation initiation to epithelial proteolysis and signaling. Exposure of TF-expressing cells to factors (F) VIIa and Xa triggered the conversion of latent pro-matriptase to an active protease, which in turn cleaved the pericellular substrates protease-activated receptor-2 (PAR2) and pro-urokinase. An activation pathway-selective PAR2 mutant resistant to direct cleavage by TF:FVIIa and FXa was activated by these proteases when cells co-expressed pro-matriptase, and matriptase transactivation was necessary for efficient cleavage and activation of wild-type PAR2 by physiological concentrations of TF:FVIIa and FXa. The coagulation initiation complex induced rapid and prolonged enhancement of the barrier function of epithelial monolayers that was dependent on matriptase transactivation and PAR2 signaling. These observations suggest that the coagulation cascade engages matriptase to help coordinate epithelial defense and repair programs after injury or infection, and that matriptase may contribute to TF-driven pathogenesis in cancer and inflammation.
Introduction
Proteases play important roles in host defense through proteolytic activation cascades that help maintain the integrity of vascular and epithelial barriers and trap and kill invading pathogens.1-4 Blood-borne proteases coordinate hemostatic and innate immune responses through coagulation and complement cascades.3,5 Under physiological conditions, the coagulation cascade is triggered by exposure of extravascular cells expressing the membrane-bound cofactor tissue factor (TF) to plasma coagulation zymogens upon vascular injury.5,6 TF-expressing cells, abundant in vascular adventitia, epithelial barriers, myocardium, and cerebral cortex, form a protective hemostatic “envelope” around blood vessels and essential organs.7 Recent observations suggest that a family of serine proteases anchored to epithelial membranes by transmembrane or glycosylphosphatidylinositol moieties (membrane-anchored serine proteases; MASPs) plays key roles in the development and homeostasis of tissue barriers.2,8-10 The activation of epithelial MASPs during organ development has been partly elucidated, but little is known about what external cues trigger their activation in mature epithelia, where they are coexpressed in latent forms or in complex with inhibitors. MASPs can be activated by serum11 and may be engaged after tissue injury to help reestablish epithelial barriers.
Although the primary role of the coagulation cascade is to prevent bleeding, blood coagulation also contributes to host defense, and coagulation proteases may initiate tissue repair programs and recruitment of immune cells by directly activating cells at the site of injury or infection.1,12 Activation of protease-activated receptors (PARs), a family of 4 G protein-coupled receptors that are activated by tethered ligands exposed by proteolytic removal of an amino-terminal exodomain, constitutes an important mechanism by which coagulation proteases modulate cellular behavior.13 On contact with plasma, TF binds factor VII (FVII)/FVIIa; TF:FVIIa activates FX to FXa, which, assisted by cofactor FVa, converts prothrombin to thrombin. Thrombin cleaves protease-activated receptor-1 (PAR1), PAR3, and PAR4 to activate platelets and endothelial cells. PAR2 can be activated by TF:FVIIa, FXa, or the ternary TF:FVIIa:FXa complex, but not by thrombin,14-16 PAR2 also transmits cellular responses to trypsin, tryptases, MASPs, kallikreins, and pathogen- and allergen-derived proteases and may constitute a form of danger receptor.17-20 PAR2 is expressed on epithelial, endothelial, smooth muscle, and immune cells and mediates epithelial fusion, inflammation, itch, and pain sensation.17,21-25 Cellular responses to PAR2 activation may also link coagulation initiation to pathological angiogenesis, cancer, diet-induced obesity, and sickle cell disease.21,26-30
Mice deficient in MASPs matriptase, prostasin, or Transmembrane Protease, Serine 13 (TMPRSS13) do not develop functional skin barriers and suffer dehydration.31-33 Tissue-specific deficiency of matriptase in intestinal epithelium leads to severe wasting and death,34 whereas loss of prostasin in alveolar epithelium impairs fluid clearance from lung.35 Deregulation of MASP activities can have equally severe consequences, as evidenced in mice by deletion of the genes encoding for their endogenous inhibitors, transmembrane hepatocyte growth factor activator inhibitor-1 (HAI-1) or HAI-2.36-41 HAI-1 supports both intracellular trafficking and proteolytic maturation of matriptase before engaging its inhibitory function10,42 and is also an inhibitor of prostasin and hepsin, which are linked with matriptase in a proteolytic cascade.21,43 Targets of this MASP cascade identified in vitro include the epithelial sodium channel (ENaC), hepatocyte growth factor (HGF), urokinase plasminogen activator (uPA), and PAR2.2 During development of the murine embryo, PAR2 helps coordinate fusion of the surface ectoderm to close the hindbrain neuropore.21 A screen for serine proteases compatible with PAR2 activation identified a cluster of MASPs coexpressed with PAR2 in surface ectoderm at the time of neural tube closure. Recombinant, soluble forms of 3 of these (matriptase, prostasin, and hepsin) induce PAR2 signaling, although hepsin and prostasin achieve PAR2 cleavage indirectly through activation of pro-matriptase.21 Matriptase is an extremely potent PAR2 agonist and is highly specific for PAR2 over other PARs.21 Subsequent studies have revealed a critical role for PAR2 in pathology associated with transgenic MASP expression.44,45
We here describe how experiments designed to address the relative importance of coagulation proteases and MASPs for PAR2 activation led us to the surprise discovery that these pathways converge on matriptase. TF:FVIIa and FXa activate pro-matriptase, which links these proteases to matriptase substrates including PAR2. This positions matriptase as a central coordinator of epithelial PAR2 activation downstream of coagulation and other proteases and suggests that the coagulation cascade may engage MASPs to initiate epithelial repair and defense pathways.
Methods
Cloning
Secreted alkaline phosphatase (SEAP)-tagged versions of human PAR1 (UniProtKB:P25116; fused at E30) and PAR2 (UniProtKB:P55085; fused at Q27) were generated for quantification of receptor cleavage.46 SEAP-PAR2 mutants targeting amino acids of the P position of the cleavage site (R36G, G35K, G35I, and G35E) were generated by polymerase chain reaction fusion of 5′ and 3′ fragments. Wild-type human prostasin (UniProtKB:Q16651), hepsin (UniProtKB:P05981), testisin/Prss21 (UniProtKB:Q9Y6M0), and matriptase (UniProtKB:Q9Y5Y6) and an inactive version of matriptase carrying a mutation in the catalytic triad (S>A) were generated by high-fidelity PCR, and all clones were fully sequenced. Expression vectors for HAI-1 and TF have been described.21,47
Cleavage assay of PAR proteins
Cells were washed for 1 hour with OptiMEM and incubated for 30 or 60 minutes with proteases and inhibitors, or for 4 hours with vehicle only, as indicated. Trypsin (10 nM for 20 minutes) was then used to strip the remaining SEAP moieties from the cell surface, and SEAP hydrolysis of para-nitrophenyl phosphate in conditioned media was assessed at OD405. Active proteolysis is reflected by an increase in percentage cleavage relative to unstimulated control wells. The response to stimulus was set to 100% when comparing the effect of serine protease inhibitors. Separate experiments confirmed that all surface-exposed SEAP-PAR proteins were cleaved during the first 10 to 15 minutes of incubation with 10 nM trypsin.
Transepithelial electrical resistance measurement
HaCaT or MCF7 cells were plated in culture dishes containing gold electrodes. Twenty-four hours after plating (or 48 hours in case of transfection), cells were washed twice with OptiMEM and resistance recorded at 500 Hz with an ECIS θ (Applied Biophysics) to monitor stabilization. Once baseline resistance had been reached, inhibitor or vehicle control was added as a fifth of the initial incubation volume. Fifteen minutes later, OptiMEM containing agonist and supplemented with inhibitor or vehicle, where appropriate, was added as a sixth of the initial incubation volume.
Results
Identification of an activation pathway-selective PAR2 mutant
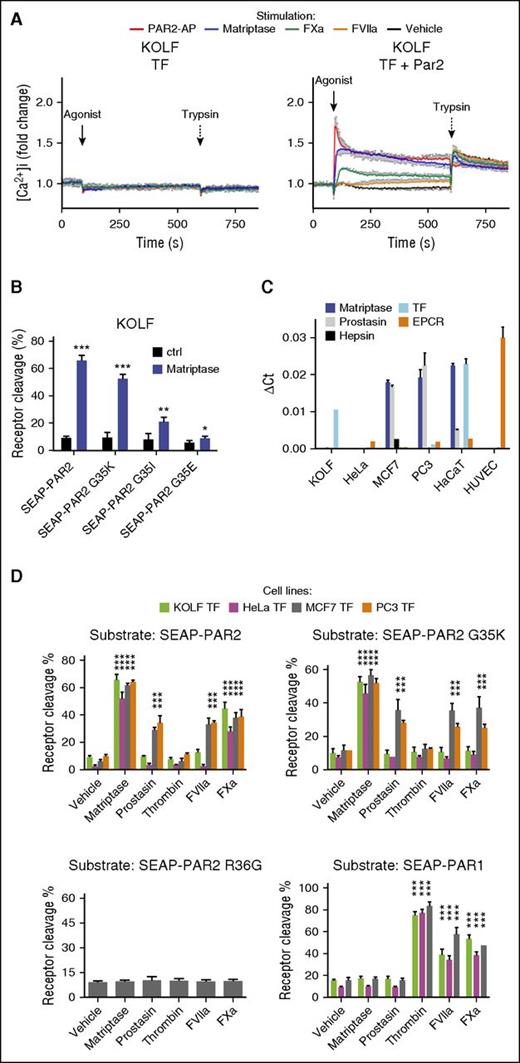
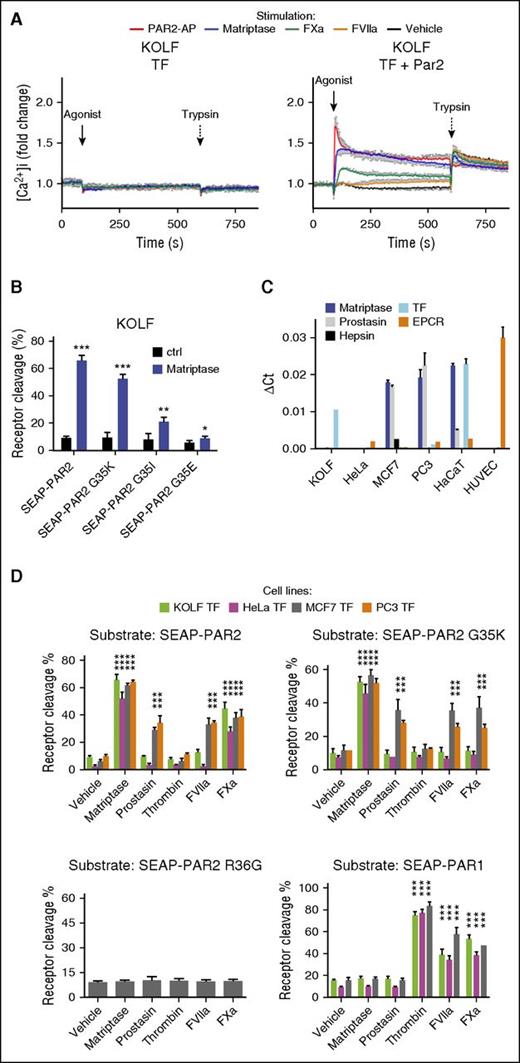
FVIIa, FXa, and matriptase all have the capacity to induce PAR2 signaling.14,16,20,21 Using changes in cytosolic free calcium as an immediate readout for PAR2 activation in PAR1 knockout lung fibroblasts (KOLFs)48 transfected with TF to allow pericellular activity of FVIIa,14,15 we confirm that matriptase is a particularly potent PAR2 agonist21 : 3 nM of the recombinant catalytic domain of human matriptase (rh-matriptase) triggered a PAR2-dependent increase in intracellular calcium equivalent to saturating concentration of a synthetic PAR2 agonist peptide (PAR2-AP) and far more robust than supra-physiological concentrations of the coagulation proteases (Figure 1A). Direct determination of cleavage efficiency performed in parallel experiments by quantification of the release of an amino terminal SEAP tag from a transfected PAR2 construct suggested that less than 2% of surface receptors were cleaved when maximal calcium responses were observed for matriptase and Xa (supplemental Figure 1, available on the Blood Web site). Responses to FVIIa were weak and not reproducibly above detection limits in either assay.
PAR2G35K is selectively cleaved by matriptase over coagulation proteases in a cell type-dependent manner. (A) KOLF cells transfected to express TF alone (left) or together with PAR2 (right) were loaded with the fluorescent calcium dye Fura2-AM and then stimulated with PAR2-AP (100 μM), matriptase (3 nM), FVIIa (10 nM), or FXa (20 nM), followed by trypsin (10 nM). Changes in intracellular calcium were calculated as described in “Methods” and normalized to the fluorescence ratio prestimulation. Note the potency of matriptase. The graph (mean ± SEM) is representative of 3 separate experiments performed in duplicate. (B) KOLFs transfected to express G35-point mutants of SEAP-PAR2 were stimulated for 30 min with matriptase (1 nM) and percentage cleavage determined as detailed in “Methods,” after a trypsin strip of remaining surface SEAP moieties. Of the 3 coagulation factor-resistant PAR2 mutants tested, only PAR2G35K remained a good substrate for recombinant matriptase. (C) Evaluation of the expression levels of selected MASPs and coagulation cofactors in selected cell lines relative to glyceraldehyde-3-phosphate dehydrogenase by quantitative reverse transcription polymerase chain reaction with species-specific primers. Note that KOLF quantification does not include EPCR. (D) HeLa, MCF7, KOLF, and PC3 cells transfected to express TF and SEAP-PAR1, SEAP-PAR2, SEAP-PAR2G35K, or SEAP-PAR2R36G were stimulated with matriptase (1 nM), prostasin (5 nM), thrombin (3 nM), FVIIa (20 nM), or FXa (40 nM) for 30 min and percentage receptor cleavage assessed. Note that SEAP-PAR2G35K is cleaved by matriptase but resistant to coagulation proteases and to prostasin, a known activator of pro-matriptase, in HeLa and KOLF cells, but not MCF7 and PC3 cells. This cleavage reflects canonical activation, as indicated by the lack of processing of SEAP-PAR2R36G. Thrombin did not cleave any PAR2 variant, but efficiently processed SEAP-PAR1. Statistical analysis was by a Student t test in B and a 1-way ANOVA followed by Newman-Keuls multiple comparison test in D. *P < .05; **P < .01; ***P < .001, relative to vehicle control.
PAR2G35K is selectively cleaved by matriptase over coagulation proteases in a cell type-dependent manner. (A) KOLF cells transfected to express TF alone (left) or together with PAR2 (right) were loaded with the fluorescent calcium dye Fura2-AM and then stimulated with PAR2-AP (100 μM), matriptase (3 nM), FVIIa (10 nM), or FXa (20 nM), followed by trypsin (10 nM). Changes in intracellular calcium were calculated as described in “Methods” and normalized to the fluorescence ratio prestimulation. Note the potency of matriptase. The graph (mean ± SEM) is representative of 3 separate experiments performed in duplicate. (B) KOLFs transfected to express G35-point mutants of SEAP-PAR2 were stimulated for 30 min with matriptase (1 nM) and percentage cleavage determined as detailed in “Methods,” after a trypsin strip of remaining surface SEAP moieties. Of the 3 coagulation factor-resistant PAR2 mutants tested, only PAR2G35K remained a good substrate for recombinant matriptase. (C) Evaluation of the expression levels of selected MASPs and coagulation cofactors in selected cell lines relative to glyceraldehyde-3-phosphate dehydrogenase by quantitative reverse transcription polymerase chain reaction with species-specific primers. Note that KOLF quantification does not include EPCR. (D) HeLa, MCF7, KOLF, and PC3 cells transfected to express TF and SEAP-PAR1, SEAP-PAR2, SEAP-PAR2G35K, or SEAP-PAR2R36G were stimulated with matriptase (1 nM), prostasin (5 nM), thrombin (3 nM), FVIIa (20 nM), or FXa (40 nM) for 30 min and percentage receptor cleavage assessed. Note that SEAP-PAR2G35K is cleaved by matriptase but resistant to coagulation proteases and to prostasin, a known activator of pro-matriptase, in HeLa and KOLF cells, but not MCF7 and PC3 cells. This cleavage reflects canonical activation, as indicated by the lack of processing of SEAP-PAR2R36G. Thrombin did not cleave any PAR2 variant, but efficiently processed SEAP-PAR1. Statistical analysis was by a Student t test in B and a 1-way ANOVA followed by Newman-Keuls multiple comparison test in D. *P < .05; **P < .01; ***P < .001, relative to vehicle control.
With the aim of developing tools to address the relative importance of MASPs vs coagulation proteases for PAR2 activation, we designed a PAR2 mutant for activation pathway-selective cleavage. PAR2 cleavage by coagulation proteases is sensitive to mutations in the P2 residue (Glycine 35) immediately N-terminal to the canonical cleavage site (Arginine 36).49 As predicted from a specificity matrix for matriptase (MEROPS),41 glycine substitution for lysine in the P2 position of PAR2 (PAR2G35K), known to induce resistance to FVIIa and FXa cleavage,49 did not impair cleavage by rh-matriptase in KOLF cells (Figure 1B). In contrast, a substantial reduction in cleavage was observed with substitutions for isoleucine or glutamic acid in the same position (PAR2G35I and PAR2G35E; Figure 1B). To confirm resistance to coagulation proteases and ensure that the ability of the recombinant catalytic domain of matriptase to recognize and cleave PAR2G35K was mirrored by the endogenous, membrane-anchored protease, cleavage was further evaluated in cells with (MCF7 and PC3) and without (KOLF and HeLa) constitutive expression of latent matriptase zymogen (Figure 1C),50 all transfected with TF. Expression of endothelial protein C receptor (EPCR), an additional cofactor for the ternary complex,51-53 was abundant in human umbilical vein endothelial cells but low or absent in the epithelial cell lines (Figure 1C). As predicted, recombinant versions of prostasin and hepsin, MASPs that use membrane-associated matriptase as an intermediate for PAR2 signaling,21 both induced PAR2 cleavage only in the pro-matriptase expressing MCF7 and PC3 cells (Figure 1D; supplemental Figure 2; and not shown).21 The same cleavage profile was observed for PAR2G35K, suggesting this mutant was efficiently cleaved by membrane-associated as well as recombinant matriptase. In contrast, and consistent with Larsen et al,49 PAR2G35K was fully resistant to cleavage by FVIIa and FXa on both HeLa and KOLF, despite TF coexpression (Figure 1D).
To our surprise, however, both FVIIa and FXa induced robust cleavage of PAR2G35K in MCF7 and PC3 cells, which also supported remarkably robust cleavage of wild-type PAR2 by FVIIa (Figure 1D). The cleavage profile of FVIIa was indistinguishable from the matriptase activators prostasin and hepsin, hinting at a similar indirect cleavage mechanism (Figure 1D; supplemental Figure 2). Neither MASPs nor coagulation proteases induced the release of the N-terminal domain of PAR2R36G, suggesting canonical receptor cleavage for both pathways. Unlike PAR2, PAR1 was efficiently cleaved by all coagulation proteases (FVIIa, FXa, and thrombin), but was fully resistant to all MASPs (prostasin, matriptase, and hepsin) tested in all 4 cell lines (hepsin in MCF7 only).
These results suggest that efficient activation of PAR2 by TF:FVIIa relies on an indirect activation mechanism that can also be engaged by FXa, and that PAR2G35K is only resistant to FVIIa and FXa in cells that do not support this mechanism.
Coagulation proteases FVIIa and FXa transactivate matriptase for PAR2G35K and PAR2 cleavage in epithelial cells
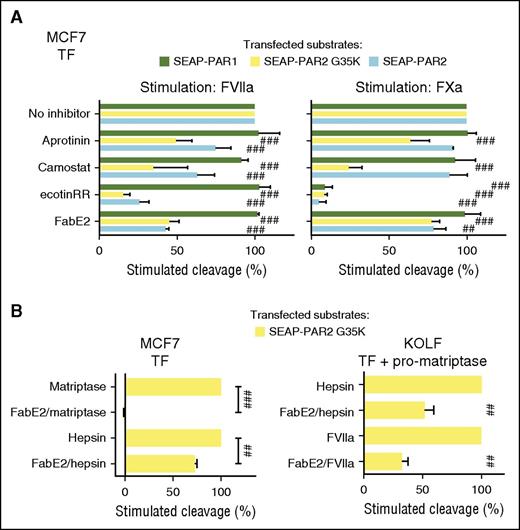
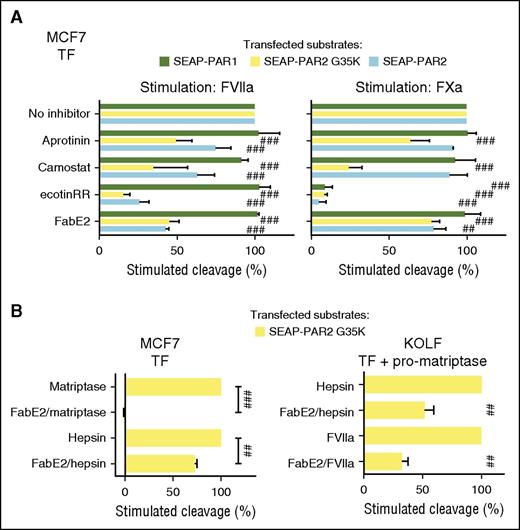
The most apparent explanation for these observations is that the coagulation proteases, in the same way as recombinant prostasin and hepsin, transactivate matriptase or another MASP to mediate and amplify cleavage of PAR2G35K and PAR2, respectively. To address this possibility and identify the putative protease intermediate, we first identified 3 protease inhibitors selective for MASPs over coagulation proteases. In a cell-free system, ecotinRR, camostat, and aprotinin all efficiently blocked the activities of recombinant matriptase and hepsin, whereas only ecotinRR inhibited FXa (supplemental Figure 3A). Prostasin was relatively resistant to camostat but sensitive to ecotinRR and aprotinin. Although we predicted cleavage of PAR2G35K by coagulation proteases to be indirect, and thus sensitive to MASP inhibitors, we predicted cleavage of PAR1 to be direct, and thus resistant to the same inhibitors. As expected for direct cleavage, sensitivity of coagulation factor stimulated-cleavage of PAR1 (Figure 2A) mirrored that of cleavage of small chromogenic substrates in the cell-free system (supplemental Figure 3A). FXa-induced cleavage of PAR1 was resistant to aprotinin and camostat, but not ecotinRR (Figure 2A). FVIIa, not tested in the cell-free system, induced full cleavage of PAR1 in the presence of all 3 inhibitors. In direct contrast, but consistent with the need for MASP transactivation, FVIIa- and FXa-induced cleavage of PAR2G35K was sensitive to all 3 inhibitors (Figure 2A). FabE2,54 an anti-matriptase antibody fragment that fully blocked the activity of rh-matriptase in cell-free assays (supplemental Figure 3B), also inhibited coagulation factor-induced PAR2G35K cleavage (Figure 2A). Arguing for a role for matriptase activation also in cleavage of the wild-type receptor, a similar inhibition profile was observed for cleavage of wild-type PAR2, although inhibition was less pronounced for FXa than for FVIIa.
MASP transactivation facilitates PAR2 cleavage by coagulation proteases. (A) MCF7 cells transfected to express TF and SEAP-PAR1, SEAP-PAR2, or SEAP-PAR2G35K were stimulated for 1 hour with either FVIIa (20 nM) or FXa (40 nM), with or without serine protease inhibitors aprotinin (1 µM), camostat (1 µM), or ecotinRR (20 nM), or the matriptase active-site blocking antibody fragment FabE2 (500 nM). Receptor cleavage was determined after a trypsin strip, unstimulated control subtracted, and uninhibited stimulation set to 100% to facilitate comparison. SEAP-PAR1 cleavage suggests FVIIa is resistant to all inhibitors and FXa to all but ecotinRR. The contrasting sensitivity to the same inhibitors for cleavage of SEAP-PAR2 variants suggests the engagement of an intermediate serine protease, probably matriptase. (B) MCF7 (left) or KOLF (right) cells transfected to express TF and SEAP-PAR2G35K with (right) or without (left) pro-matriptase zymogen were stimulated for 1 hour with matriptase (3 nM), hepsin (10 nM), or FVIIa (20 nM) with or without FabE2 (500 nM) and cleavage inhibition determined as in A. Note that despite potent inhibition of PAR2G35K cleavage by the soluble catalytic domain of recombinant matriptase, FabE2 does not fully inhibit PAR2G35K cleavage by membrane-associated matriptase elicited by FVIIa or hepsin in either a natural (left) or a fully reconstituted (right) system. Statistical analysis was by 1-way ANOVA followed by Newman-Keuls multiple comparison test. ##P < .01; ###P < .001 relative to uninhibited.
MASP transactivation facilitates PAR2 cleavage by coagulation proteases. (A) MCF7 cells transfected to express TF and SEAP-PAR1, SEAP-PAR2, or SEAP-PAR2G35K were stimulated for 1 hour with either FVIIa (20 nM) or FXa (40 nM), with or without serine protease inhibitors aprotinin (1 µM), camostat (1 µM), or ecotinRR (20 nM), or the matriptase active-site blocking antibody fragment FabE2 (500 nM). Receptor cleavage was determined after a trypsin strip, unstimulated control subtracted, and uninhibited stimulation set to 100% to facilitate comparison. SEAP-PAR1 cleavage suggests FVIIa is resistant to all inhibitors and FXa to all but ecotinRR. The contrasting sensitivity to the same inhibitors for cleavage of SEAP-PAR2 variants suggests the engagement of an intermediate serine protease, probably matriptase. (B) MCF7 (left) or KOLF (right) cells transfected to express TF and SEAP-PAR2G35K with (right) or without (left) pro-matriptase zymogen were stimulated for 1 hour with matriptase (3 nM), hepsin (10 nM), or FVIIa (20 nM) with or without FabE2 (500 nM) and cleavage inhibition determined as in A. Note that despite potent inhibition of PAR2G35K cleavage by the soluble catalytic domain of recombinant matriptase, FabE2 does not fully inhibit PAR2G35K cleavage by membrane-associated matriptase elicited by FVIIa or hepsin in either a natural (left) or a fully reconstituted (right) system. Statistical analysis was by 1-way ANOVA followed by Newman-Keuls multiple comparison test. ##P < .01; ###P < .001 relative to uninhibited.
Inhibition profiles for FVIIa-induced cleavage of wild-type PAR2 and PAR2G35K were similar in pro-matriptase-expressing MCF7 cells, even if PAR2G35K is fully resistant to cleavage in KOLF and HeLa cells (Figure 1D). This argues that residual cleavage after MASP inhibition is more likely to reflect on incomplete MASP inhibition than direct processing of the wild-type receptor by FVIIa. To test this more directly, we assessed the ability of FabE2 to inhibit PAR2 cleavage by recombinant hepsin, which is known to require matriptase activation.21 Although cleavage of PAR2G35K by the recombinant soluble catalytic domain of matriptase was fully inhibited with 500 nM FabE2, the same concentration of antibody showed significant but much less profound inhibition of hepsin-induced cleavage of PAR2G35K (Figure 2B). Similar significant but incomplete inhibition of hepsin- or FVIIa-induced cleavage was observed with FabE2 in KOLF cells reconstituted with TF, pro-matriptase, and PAR2G35K (Figure 2B), even if no PAR2G35K cleavage by FVIIa was observed in KOLF cells without coexpression of pro-matriptase (Figure 1D). We interpret this to suggest that temporal and/or spatial constraints in access to the active site of matriptase between zymogen conversion and receptor cleavage precludes complete inhibition of PAR2 activation. Of note, camostat and aprotinin did not affect the overall kinetics of thrombin generation in TF-transfected HeLa and MCF7 cells (supplemental Figure 4A-C), arguing that matriptase inhibition can be achieved without impairing essential hemostasis secured by the TF pathway.
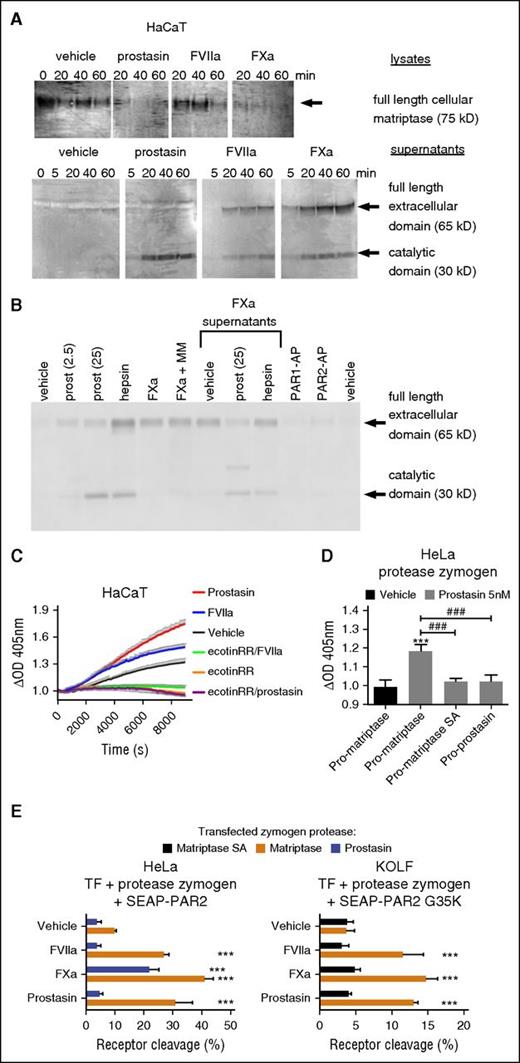
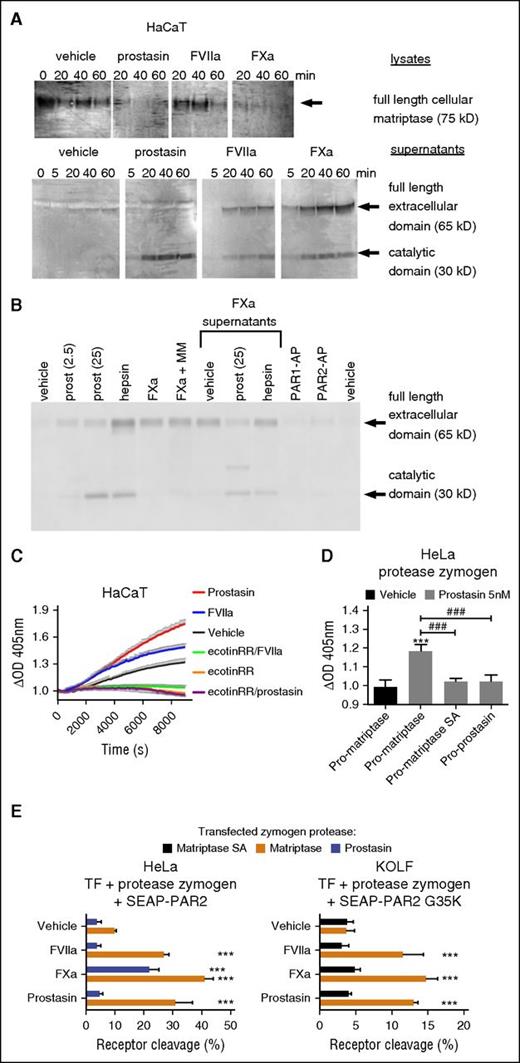
We next addressed whether FVIIa and FXa can process pro-matriptase in HaCaT human keratinocytes, which endogenously express TF and pro-matriptase, as well as PAR2 (supplemental Figure 1C).15,21 As previously demonstrated,21 both rh-hepsin and rh-prostasin triggered loss of cell-associated matriptase zymogen and accumulation of active matriptase in HaCaT supernatants (Figure 3A; supplemental Figure 5A), consistent with zymogen conversion. Intriguingly, both TF:FVIIa and FXa were also able to trigger shedding and activation of endogenous or transfected matriptase (Figure 3A; supplemental Figure 5B-C). Shedding was independent of PAR activation and of the zinc-metalloprotease axis and did not depend on the activity of matriptase itself (Figure 3B; supplemental Figure 5B-C). The intact extracellular domain of matriptase appeared more prominent than the catalytic domain in supernatants of cells treated with coagulation proteases (Figure 3A), in part because the recombinant MASPs, and prostasin in particular, continued to process matriptase after its release into the supernatant (Figure 3B). To confirm that matriptase processing by coagulation proteases correlated with increased cell surface MASP activity, we monitored cleavage of the chromogenic substrate S2765 on HaCaT, MCF7, and HeLa cells after the addition of soluble proteases. Prostasin stimulated hydrolysis of S2765 on HaCaT (Figure 3C) and MCF7 (supplemental Figure 5D), 2 cell lines that constitutively express pro-matriptase. Similar rh-prostasin-induced hydrolysis was achieved on HeLa cells on transfection with wild-type pro-matriptase, but neither by catalytically inactive pro-matriptase nor by wild-type prostasin zymogen (Figure 3D). Consistent with activation of matriptase, TF:FVIIa also stimulated hydrolysis of S2765 in both HaCaT (Figure 3C) and MCF7 (supplemental Figure 5D), a substantial component of which could be inhibited by the MASP-selective inhibitor ecotinRR (which also suppressed basal matriptase-like activity).
Coagulation proteases transactivate latent matriptase to enhance epithelial PAR2 cleavage. (A) HaCaT cells were incubated for the indicated time with prostasin (25 nM), FVIIa (25 nM), or FXa (50 nM) cell lysates, and concentrated supernatants were denatured, size-separated, blotted, and probed with an anti-matriptase antibody. Note the consumption of cell-associated matriptase and corresponding accumulation of the extracellular (by FVIIa and FXa only) and catalytic (by all 3 proteases) domains in supernatants. The blot is representative of 4 independent experiments (quantification in supplemental Figure 5A). (B) HaCaT cells were incubated for 1 hour with prostasin (2.5 or 25 nM), hepsin (30 nM), FXa (50 nM), PAR1-AP (50 µM), or PAR2-AP (50 µM), either alone or supplemented with marimastat (MM, 1 µM). Where indicated, cell-free supernatants of FXa-treated cells were incubated for an additional hour with no further addition of protease or after supplementation of prostasin (25 nM) or hepsin (30 nM). Samples were denatured, size-separated, blotted, probed with an anti-matriptase antibody, and revealed. Note that the full-length extracellular domain released by Xa can be further matured in solution by prostasin and that shedding is independent of zinc metalloproteases and does not require engagement of PARs. (C) Activation of serine proteases with matriptase-like specificity was studied by stimulation of HaCaT cells with prostasin (5 nM) or FVIIa (20 nM) in the presence of the chromogenic substrate S2765. OD405 values were normalized to prestimulation values. Note that prostasin and FVIIa both stimulate matriptase-like activity in HaCaT, which also shows constitutive matriptase-like activity. All were inhibited by the MASP inhibitor ecotinRR (20 nM). The graph (mean ± SEM) is representative of 3 separate experiments performed in triplicate. (D) HeLa cells transfected with zymogens of wild-type matriptase, catalytically inactive matriptase, or prostasin were used to determine the cell surface activity of serine proteases against the chromogenic substrate S2765 upon stimulation with soluble prostasin. Note that only transfection of wild-type pro-matriptase yields a net increase in OD405 after stimulation with prostasin for 3.5 hours. (E) HeLa (left) and KOLF (right) cells were transfected to express TF, SEAP-PAR2, or SEAP-PAR2G35K together with candidate protease zymogen, as indicated, and stimulated with FVIIa (20 nM), FXa (40 nM), or prostasin (5 nM) for 1 hour, followed by a trypsin strip for determination of receptor cleavage. Only wild-type matriptase zymogen permitted (FVIIa, prostasin) or enhanced (FXa) receptor cleavage. Pooled data ± SD of 4 independent experiments performed in duplicate.
Coagulation proteases transactivate latent matriptase to enhance epithelial PAR2 cleavage. (A) HaCaT cells were incubated for the indicated time with prostasin (25 nM), FVIIa (25 nM), or FXa (50 nM) cell lysates, and concentrated supernatants were denatured, size-separated, blotted, and probed with an anti-matriptase antibody. Note the consumption of cell-associated matriptase and corresponding accumulation of the extracellular (by FVIIa and FXa only) and catalytic (by all 3 proteases) domains in supernatants. The blot is representative of 4 independent experiments (quantification in supplemental Figure 5A). (B) HaCaT cells were incubated for 1 hour with prostasin (2.5 or 25 nM), hepsin (30 nM), FXa (50 nM), PAR1-AP (50 µM), or PAR2-AP (50 µM), either alone or supplemented with marimastat (MM, 1 µM). Where indicated, cell-free supernatants of FXa-treated cells were incubated for an additional hour with no further addition of protease or after supplementation of prostasin (25 nM) or hepsin (30 nM). Samples were denatured, size-separated, blotted, probed with an anti-matriptase antibody, and revealed. Note that the full-length extracellular domain released by Xa can be further matured in solution by prostasin and that shedding is independent of zinc metalloproteases and does not require engagement of PARs. (C) Activation of serine proteases with matriptase-like specificity was studied by stimulation of HaCaT cells with prostasin (5 nM) or FVIIa (20 nM) in the presence of the chromogenic substrate S2765. OD405 values were normalized to prestimulation values. Note that prostasin and FVIIa both stimulate matriptase-like activity in HaCaT, which also shows constitutive matriptase-like activity. All were inhibited by the MASP inhibitor ecotinRR (20 nM). The graph (mean ± SEM) is representative of 3 separate experiments performed in triplicate. (D) HeLa cells transfected with zymogens of wild-type matriptase, catalytically inactive matriptase, or prostasin were used to determine the cell surface activity of serine proteases against the chromogenic substrate S2765 upon stimulation with soluble prostasin. Note that only transfection of wild-type pro-matriptase yields a net increase in OD405 after stimulation with prostasin for 3.5 hours. (E) HeLa (left) and KOLF (right) cells were transfected to express TF, SEAP-PAR2, or SEAP-PAR2G35K together with candidate protease zymogen, as indicated, and stimulated with FVIIa (20 nM), FXa (40 nM), or prostasin (5 nM) for 1 hour, followed by a trypsin strip for determination of receptor cleavage. Only wild-type matriptase zymogen permitted (FVIIa, prostasin) or enhanced (FXa) receptor cleavage. Pooled data ± SD of 4 independent experiments performed in duplicate.
TF:FVIIa and FXa-induced proteolytic activation of endogenous matriptase in HaCaT and MCF7 cells is not necessarily direct and could involve an endogenous matriptase-activating protease such as prostasin (Figure 1C).46 To address this possibility and to further establish the capacity of pro-matriptase to support activation of PAR2 by coagulation proteases, we reconstituted the theoretical signaling cascade in HeLa and KOLF cells, which express little if any endogenous matriptase, prostasin, or hepsin (Figure 1C). Consistent with direct activation of matriptase, coexpression of wild-type matriptase zymogen, but neither catalytically inactive pro-matriptase nor proforms of prostasin, hepsin, or testisin/Prss21 (another MASP with PAR2 processing activity55 ), facilitated FVIIa- and prostasin-induced cleavage of PAR2 and FVIIa-, FXa-, and prostasin-mediated cleavage of PAR2G35K in HeLa and KOLF cells (Figure 3E; supplemental Figure 6).
Collectively, these biochemical observations argue that FVIIa and FXa both activate matriptase, and that such MASP transactivation substantially amplifies PAR2 cleavage by coagulation proteases in cells that express pro-matriptase; that is, most PAR2-expressing epithelia.21
Matriptase amplifies PAR2 signaling by coagulation proteases
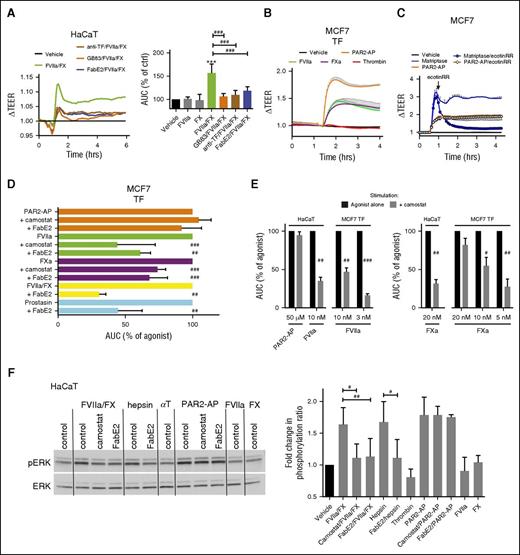
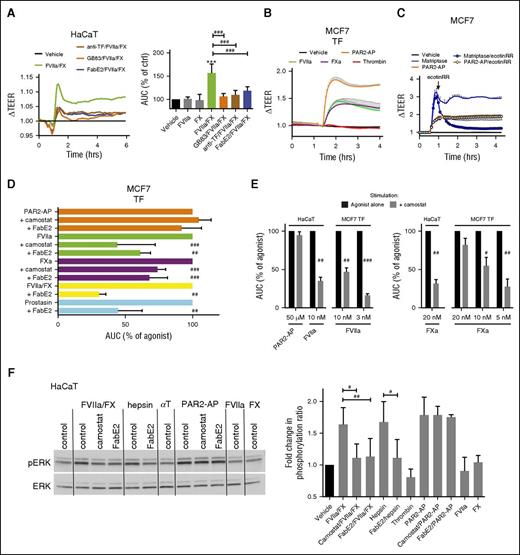
Sensitivity to mutations at the canonical cleavage site argues that observed cleavage efficiencies reflect directly on PAR2 activation. To validate this assumption, we first addressed the importance of matriptase transactivation for acute PAR2-dependent behavioral response to coagulation proteases reflected in changes in transepithelial electrical resistance (TEER) by electric cell-substrate impedance sensing.32,34 Both direct and indirect PAR2 agonists increased TEER of HaCaT and MCF7-TF monolayers (Figure 4A-B; supplemental Figure 7A). Responses to matriptase were rapidly reversible with ecotinRR, suggesting the prolonged increase in TEER was continuously sustained by receptor activation (Figure 4C; supplemental Figure 7B). Consistent with an important role for matriptase transactivation, responses to prostasin, hepsin, FVIIa, FXa, and FVIIa/FX (for ternary complex signaling) were consistently inhibited by camostat or FabE2 in HaCaT and MCF7 cells (Figure 4A,D). The relative effect of MASP inhibition with camostat suggested that matriptase transactivation plays a greater role at lower doses of coagulation proteases and a more important role in HaCaT than MCF7 (Figure 4E). Residual signaling in the presence of MASP inhibitors is likely to reflect incomplete matriptase inhibition, as well as direct PAR2 cleavage at higher concentrations of stimulating protease. The requirement for TF and PAR2 in the response to coagulation proteases was confirmed with a TF-blocking antibody and a PAR2 antagonist (GB-83; Figure 4A; supplemental Figure 7C-D). An equally profound effect of matriptase inhibition was observed on the capacity of the coagulation proteases to induce PAR2-dependent ERK phosphorylation in HaCaT cells (Figure 4F). No effect of thrombin on TEER or pERK abundance (Figure 4B,F; supplemental Figure 7A) argues that PAR2 activation triggered by proteases of the extrinsic cascade dominates coagulation signaling, at least in these epithelial cell lines. Together, these observations suggest that matriptase is an important mediator and amplifier of epithelial responses to coagulation proteases.
Matriptase mediates epithelial signaling induced by the coagulation initiation complex. (A) HaCaT cells were incubated for 1 hour in the presence of anti-TF (4.5 µg/mL), GB83 (20 µM), or FabE2 (500 nM), and changes in TEER were recorded after the addition of FVIIa (50 pM)/X (100 nM). (Left) Representative tracings normalized to prestimulation values and vehicle control. (Right) Area under the curve (AUC) (mean ± SD of 4 independent experiments). Note that all inhibitors blunted the ability of coagulation proteases to increase TEER. (B) TF-transfected MCF7 cells were stimulated with the matriptase activator prostasin (5 nM), PAR2-AP (50 μM), FVIIa (10 nM), FXa (20 nM), or thrombin (5 nM), and TEER was recorded. Note that the PAR2-AP and all proteases but thrombin induce a prolonged increase in barrier function. (C) MCF7 cells were incubated with matriptase (0.5 nM) or PAR2-AP (50 μM), and TEER was recorded. When TEER peaked in response to matriptase, the serine protease inhibitor ecotinRR (50 nM) or vehicle control was added (arrow). Note the rapid return to baseline on inhibition of matriptase, suggesting that continuous receptor activation sustains high resistance. The graph is representative of 3 independent experiments (quantification in supplemental Figure 7B). Tracings in B and C are normalized to prestimulation values and vehicle control. (D) TF-transfected MCF7 cells were incubated with camostat (1 μM), FabE2 (500 nM), or vehicle for 15 min before the addition of agonists PAR2-AP (50 μM), FVIIa (10 nM), FXa (20 nM), FVIIa (50 pM)/FX (100 nM), or prostasin (5 nM), and TEER was recorded during 4 hours. The AUC was quantified and expressed as a percentage of the unrestrained agonist response. Camostat and FabE2 significantly inhibited responses to the matriptase-activator prostasin and to coagulation proteases, but not to PAR2-AP. (E) TF-transfected MCF7 or HaCaT cells were incubated with camostat (1 μM) or vehicle for 15 min before addition of agonists, and TEER was recorded during 4 hours. The AUC was quantified and expressed as a percentage of the unrestrained agonist response. Responses are more sensitive to camostat at low concentrations of coagulation proteases and are more sensitive in HaCaT than MCF7. (F) HaCaT cells were incubated with camostat (1 μM), FabE2 (500 nM), or vehicle for 10 min before stimulation for 10 min with FVIIa (50 pM) and/or FX (100 nM), hepsin (10 nM), thrombin (αT, 5 nM), or PAR2-AP (50 µM). Total ERK and phospho-ERK levels were determined by western blotting. (Left) Representative experiment. (Right) Pooled ratiometric analyses of pERK/total ERK (mean ± SD; n = 3). Note that all agonists except thrombin induced an increase in ERK phosphorylation and that activation by proteases, but not peptide agonist, was sensitive to matriptase inhibitors. After 1-way ANOVA, a Newman-Keuls multiple comparison test was used for statistical analysis in A and for PAR2-AP, FVIIa, and FXa stimulations in D; Student t test was used for analysis of the data elsewhere. ***P < .001 relative to vehicle control; #P < .05, ##P < .01, ###P < .001 relative to uninhibited.
Matriptase mediates epithelial signaling induced by the coagulation initiation complex. (A) HaCaT cells were incubated for 1 hour in the presence of anti-TF (4.5 µg/mL), GB83 (20 µM), or FabE2 (500 nM), and changes in TEER were recorded after the addition of FVIIa (50 pM)/X (100 nM). (Left) Representative tracings normalized to prestimulation values and vehicle control. (Right) Area under the curve (AUC) (mean ± SD of 4 independent experiments). Note that all inhibitors blunted the ability of coagulation proteases to increase TEER. (B) TF-transfected MCF7 cells were stimulated with the matriptase activator prostasin (5 nM), PAR2-AP (50 μM), FVIIa (10 nM), FXa (20 nM), or thrombin (5 nM), and TEER was recorded. Note that the PAR2-AP and all proteases but thrombin induce a prolonged increase in barrier function. (C) MCF7 cells were incubated with matriptase (0.5 nM) or PAR2-AP (50 μM), and TEER was recorded. When TEER peaked in response to matriptase, the serine protease inhibitor ecotinRR (50 nM) or vehicle control was added (arrow). Note the rapid return to baseline on inhibition of matriptase, suggesting that continuous receptor activation sustains high resistance. The graph is representative of 3 independent experiments (quantification in supplemental Figure 7B). Tracings in B and C are normalized to prestimulation values and vehicle control. (D) TF-transfected MCF7 cells were incubated with camostat (1 μM), FabE2 (500 nM), or vehicle for 15 min before the addition of agonists PAR2-AP (50 μM), FVIIa (10 nM), FXa (20 nM), FVIIa (50 pM)/FX (100 nM), or prostasin (5 nM), and TEER was recorded during 4 hours. The AUC was quantified and expressed as a percentage of the unrestrained agonist response. Camostat and FabE2 significantly inhibited responses to the matriptase-activator prostasin and to coagulation proteases, but not to PAR2-AP. (E) TF-transfected MCF7 or HaCaT cells were incubated with camostat (1 μM) or vehicle for 15 min before addition of agonists, and TEER was recorded during 4 hours. The AUC was quantified and expressed as a percentage of the unrestrained agonist response. Responses are more sensitive to camostat at low concentrations of coagulation proteases and are more sensitive in HaCaT than MCF7. (F) HaCaT cells were incubated with camostat (1 μM), FabE2 (500 nM), or vehicle for 10 min before stimulation for 10 min with FVIIa (50 pM) and/or FX (100 nM), hepsin (10 nM), thrombin (αT, 5 nM), or PAR2-AP (50 µM). Total ERK and phospho-ERK levels were determined by western blotting. (Left) Representative experiment. (Right) Pooled ratiometric analyses of pERK/total ERK (mean ± SD; n = 3). Note that all agonists except thrombin induced an increase in ERK phosphorylation and that activation by proteases, but not peptide agonist, was sensitive to matriptase inhibitors. After 1-way ANOVA, a Newman-Keuls multiple comparison test was used for statistical analysis in A and for PAR2-AP, FVIIa, and FXa stimulations in D; Student t test was used for analysis of the data elsewhere. ***P < .001 relative to vehicle control; #P < .05, ##P < .01, ###P < .001 relative to uninhibited.
Matriptase activation connects the coagulation cascade to epithelial proteolysis
If coagulation proteases transactivate latent matriptase, this should connect the coagulation cascade to the whole repertoire of matriptase substrates, not only to PAR2. To test this concept, we addressed whether FVIIa could activate the pro-form of urokinase-plasminogen-activator (single chain-uPA or scuPA), a physiological activator of plasminogen and a known matriptase substrate.2,20,56 We assayed activation of exogenously added scuPA on FVIIa- or prostasin-treated MCF7 cells in the presence or absence of aprotinin, the only matriptase inhibitor tested that did not also directly inhibit uPA (supplemental Figure 8A). Both matriptase activators rh-prostasin and FVIIa promoted scuPA conversion (supplemental Figure 8B), and sensitivity of FVIIa-induced scuPA conversion to aprotinin confirmed the involvement of a matriptase-like protease. Slow kinetics of scuPA conversion by both FVIIa and prostasin reflect on matriptase being a far less efficient activator of scuPA than plasmin.57 Matriptase expression-dependent scuPA conversion has nevertheless been demonstrated to substantially reduce the lag time for plasminogen conversion on the cell surface,57 suggesting it might have physiological relevance. More broadly, this argues that the consequences of matriptase activation by coagulation proteases are not limited to PAR2 signaling.
Discussion
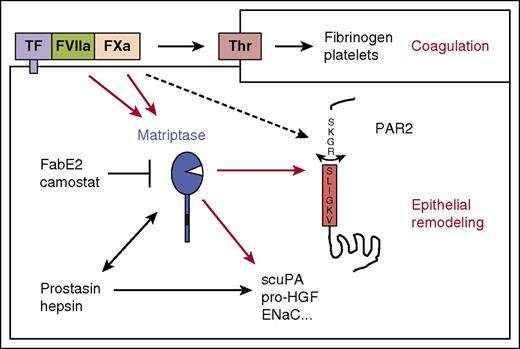
We here demonstrate that proteases of the TF-dependent (extrinsic) pathway of blood coagulation engage the membrane-anchored serine protease matriptase to modulate epithelial behavior (Figure 5). Activation of coagulation zymogens by interaction with TF on tissue injury is critical for hemostasis. Transactivation of the MASP matriptase may connect coagulation initiation to epithelial defense and repair programs and contribute to pathogenic effects of extrinsic pathway activation in cancer and inflammation.
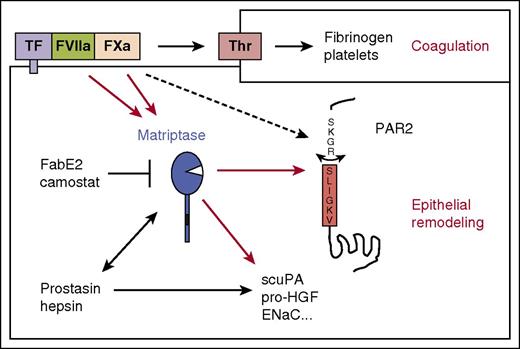
Matriptase connects the coagulation cascade to epithelial signaling and proteolysis. Schematic representation of how matriptase amplifies coagulation factor signaling in epithelia. The results of this study suggest that FVIIa and FXa both directly activate matriptase, which in turn activates PAR2 and processes other epithelial substrates. This may connect coagulation activation to epithelial remodeling. Matriptase also mediates PAR2 signaling by the epithelial MASPs hepsin and prostasin. Additional cofactors such as EPCR may support PAR2 activation by the TF:FVIIa:FXa ternary complex, and at high concentrations, FXa may directly activate PAR2 without matriptase transactivation.
Matriptase connects the coagulation cascade to epithelial signaling and proteolysis. Schematic representation of how matriptase amplifies coagulation factor signaling in epithelia. The results of this study suggest that FVIIa and FXa both directly activate matriptase, which in turn activates PAR2 and processes other epithelial substrates. This may connect coagulation activation to epithelial remodeling. Matriptase also mediates PAR2 signaling by the epithelial MASPs hepsin and prostasin. Additional cofactors such as EPCR may support PAR2 activation by the TF:FVIIa:FXa ternary complex, and at high concentrations, FXa may directly activate PAR2 without matriptase transactivation.
Cellular responses to proteases of the extrinsic pathway were originally described in epithelial cell lines, kidney epithelial cells (MDCK), urinary bladder epithelial cells (J82), and keratinocytes (HaCaT).15,58,59 Lack of responses to FVIIa in cells naturally expressing (HK-2) or induced to express (CHO) TF and PAR2 initially argued against PAR2 as the FVIIa receptor,15 but we later found TF and PAR2 to be sufficient to support FVIIa and FXa signaling in Xenopus oocytes and KOLF.14 Our current observations suggest that matriptase transactivation was missed as a key component of PAR2 signaling in these studies: TF and PAR2 dependence did not exclude the need for a protease intermediate, and reconstitution experiments involved expression of TF at levels high enough to overcome this requirement.14,60 In HaCaT keratinocytes, which naturally express all components of the signaling cascade, recombinant or purified FVIIa, FXa, and matriptase can induce cellular responses with half-maximal efficiency at 3, 13, and 0.2 nM, respectively.15,21 Our current observations suggest that coagulation factor signaling at these concentrations is highly dependent on matriptase transactivation. This indirect mechanism appears to be critical for direct PAR2 activation by TF:FVIIa, which is mainly observed in epithelia. Although it is difficult to estimate the local concentrations of FXa reached in the presence of inhibitors in vivo, they are unlikely to exceed levels at which matriptase, if present, plays an important amplifying role. Signaling in the context of the ternary TF:FVIIa:Xa complex16 is supported by EPCR in several cell types, including HaCaT.52 We observe signaling triggered by picomolar FVIIa with subplasma concentrations of FX to be highly matriptase-dependent in HaCaT and efficient in cells that do not express EPCR. This argues that matriptase is required for ternary complex signaling downstream of EPCR in epithelia and that EPCR is not essential. The importance of matriptase transactivation for coagulation signaling in any given cell type will depend on the expression of cofactors, pro-matriptase, PARs, and inhibitors. Matriptase activation is likely to be important in most epithelia because of the widespread expression of pro-matriptase and TF and the dominance of PAR2 over other PARs,7,21,61,62 but irrelevant in endothelial cells, as they express little matriptase or TF even when activated; PAR1 and PAR4, in addition to PAR2; and high levels of EPCR (Figure 1C).7,60,63 In this context, FXa may directly activate PAR2 independent of TF and contribute to PAR1 activation with thrombin and APC, and we would expect FVIIa to have minimal direct contribution to PAR2 cleavage, although it may participate as part of the ternary complex if TF is expressed, potentially supported by EPCR.14,60,63,64
Recent studies in genetically modified mice have revealed a key role for MASPs in epithelial homeostasis and disease.2,34,40,65 MASPs activate each other during the development of epithelial barriers,2,31,32,36,40,66,67 but it remains poorly understood when and how these cascades are activated in mature, polarized epithelia that harbor latent MASP zymogens. One of the first described activators of matriptase zymogen was serum.11 Although the activity in serum was attributed to sphingosine-1-phosphate,68 we observe its capacity to activate matriptase to be restricted (results not shown), arguing that coagulation proteases are more important blood-derived activators. Activation by coagulation proteases suggests epithelial MASPs are elicited in response to injury, possibly to carry out their developmental roles in coordinating the regeneration of functional epithelial barriers. This may provide a new mechanistic link between coagulation activation and tissue repair. During neurulation, epithelial PAR2 coordinates hindbrain closure.21 Transcriptional responses to FVIIa in keratinocytes suggest coagulation signaling may serve to promote tissue repair and host defense,69 and our current observation argues that other matriptase substrates may contribute with PAR2 to these and other epithelial responses to coagulation activation after injury.31,34,70-73 Rapid and prolonged increases in epithelial barrier function on matriptase transactivation by the coagulation initiation complex support this notion.
Although transactivation of MASPs by coagulation proteases is likely to have evolved as a physiological response, deregulated MASP activity and aberrant TF-dependent activation of the coagulation cascade have been independently associated with excessive inflammatory responses and cancer, in both cases implicating PAR2 signaling.27-30,44,45,65,74 Our current observations suggest these phenomena may be linked. Vascular inflammation induced by TF is unlikely to involve matriptase, but plasma leak, bleeding, or ectopic expression of FVIIa75 could trigger TF and matriptase-dependent pathology, both via and independent of PAR2. In the polyoma middle T antigen-induced breast cancer model, genetic or pharmacological inhibition of TF, matriptase, and PAR2 all blunt tumor growth.29,76-78 Matriptase plays a critical role in pro-HGF activation and c-Met signaling in this model.77 The role for coagulation proteases in matriptase activation, the importance of matriptase for PAR2 activation, and relative roles of PAR2 and HGF downstream of matriptase remain to be determined. Matriptase activation by coagulation proteases argues that pro-HGF, pro-urokinase, and other matriptase substrates should be considered alongside PARs and fibrin downstream of TF in tumor growth and dissemination.78-80 Modest pro-matriptase overexpression in skin both triggers and potentiates tumor formation.81 Intriguingly, this is critically dependent on both PAR2 and cMet, suggesting interaction between these effectors.44,72 Whether or not coagulation proteases contribute to matriptase activation in this context remains to be determined.
Collectively, our results show that activation of TF-dependent coagulation on epithelia triggers activation of the epithelial transmembrane protease matriptase and argue that matriptase effectors including PAR2 may contribute to both physiological and pathological responses to coagulation activation in epithelia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by INSERM Avenir, Marie Curie Actions (IRG-249193), the French National Research Agency (ANR-10-MIDI-0003, ANR-15-CE14-0009), the Ile-de-France Region (E.C.), the US Department of Defense PC 11318 (C.S.C.) and by the National Institute of Dental and Craniofacial Research Intramural Research Program (T.H.B.).
Authorship
Contribution: S.M.L.G. and R.S. designed and performed experiments and analyzed data; M.L., D.K., C.S.C., and T.H.B. provided reagents and conceptual advice; and S.M.L.G., R.S., and E.C. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Camerer, Inserm U970, Paris Cardiovascular Research Center, 56 Rue Leblanc, 75015 Paris, France; e-mail: eric.camerer@inserm.fr.