Key Points
Acquired MPO deficiency in patients with MPN is uniquely associated with homozygous CALR mutations.
In line with a posttranscriptional defect, MPO deficiency results from reduced MPO protein levels, but not from decreased MPO mRNA.
Abstract
The pathogenesis of acquired myeloperoxidase (MPO) deficiency, a rare phenomenon observed in patients with Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), is unknown. MPO is a glycoprotein (GP) chaperoned by calreticulin (CALR) in the endoplasmic reticulum. Mutations in CALR are frequently found in patients with myelofibrosis (MF) and essential thrombocythemia (ET) with nonmutated Janus kinase 2 (JAK2). We hypothesized that acquired MPO deficiency in MPN could be associated with the presence of CALR mutations. A cohort of 317 patients with MPN (142 polycythemia vera [PV], 94 ET, and 81 MF) was screened for MPO deficiency. MPO deficiency was observed in 6/81 MF patients (7.4%), but not in PV or ET patients. Susceptibility to infections had been documented in 2/6 (33%) MPO-deficient patients. Five out of 6 patients with MPO deficiency carried a homozygous CALR mutation and were also deficient in eosinophilic peroxidase (EPX). In contrast, 1 patient with MF, a JAK2-V617F mutation, and MPO deficiency, carried 2 previously reported MPO mutations and showed normal EPX activity. Patients with homozygous CALR mutations had reduced MPO protein, but normal MPO messenger RNA (mRNA) levels supporting a posttranscriptional defect in MPO production. Finally, we demonstrate in vitro that in the absence of CALR, immature MPO protein precursors are degraded in the proteasome. Therefore, 4 decades after the first description of acquired MPO deficiency in MPN, we provide the molecular correlate associated with this phenomenon and evidence that CALR mutations can affect the biosynthesis of GPs.
Introduction
Myeloperoxidase (MPO) is a lysosomal hemeprotein expressed exclusively in myeloid cells. MPO catalyzes the conversion from hydrogen peroxide to hypochlorous acid and is essential for optimal oxygen-dependent antimicrobial activity.1,2 In the endoplasmic reticulum (ER), glycosylated MPO precursors are chaperoned by the calreticulin (CALR)-calnexin (CNX) cycle.3-5 Both CALR and CNX are lectins that transiently bind to virtually all newly generated glycoproteins (GPs), and their specificity for GPs is mediated by a binding site that recognizes the oligosaccharide Glc1Man9GlcNAc2.5 CALR and CNX function as a quality control system in the ER before proteins are shuttled to the Golgi complex. MPO precursors that fail to mature and remain for prolonged periods in the CALR-CNX cycle are degraded in the proteasome.5,6
MPO deficiency can either be inherited or acquired. In a large study, partial MPO deficiency was detected in ∼0.2% of healthy individuals, whereas complete MPO deficiency was found only in 55/15 0000 (0.04%) study subjects.7 In the same study, an increased incidence of serious infections and inflammatory disease was reported in patients with complete MPO deficiency. The pathogenesis of acquired MPO deficiency, a rare phenomenon observed in patients with hematopoietic stem cell neoplasms, is unknown.8 In Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), MPO deficiency is most frequently detected in patients with myelofibrosis (MF), whereas the incidence is lower in polycythemia vera (PV) and essential thrombocythemia (ET).9
Mutations in CALR were recently described in the majority of patients with ET and MF with nonmutated Janus kinase 2 (JAK2).10,11 Although 36 distinct CALR mutations have been described, 2 types of mutations, type 1 and 2, cover >80% of the mutational spectrum of CALR. CALR mutations lead to a +1 base pair (bp) frameshift, which affects the C-terminal domain.10,11 Although the expression of CALR mutants in mouse bone marrow induces an ET/MF phenotype through activation of the thrombopoietin receptor (MPL), the consequences of CALR mutations on the protein function remain to be determined.12 In this study, we found that acquired MPO deficiency in MPN is associated with the presence of homozygous CALR mutations.
Materials and methods
Patients and samples
We investigated patients with MF (primary MF, post-ET MF, and post-polycythemia MF), ET, and PV according to the 2008 World Health Organization classification, who were regularly seen at the University Hospital Zurich and the University Hospital Basel, Switzerland. The study was approved by the Kantonale Ethikkommission Zurich (cantonal ethics committee), and all study patients provided written informed consent.
Automated cell count with the ADVIA 2120
Automated cell count by the ADVIA 2120 was performed as described.13
Flow cytometry
Analysis of purified neutrophil granulocytes and peripheral blood mononuclear cells was performed on a Becton Dickinson FACS Canto and LSRFortessa. Cells were fixed and permeabilized with a kit from Beckman Coulter. Cells were stained with the following antibodies: phycoerythrin-conjugated anti-CALR (Abcam); fluorescein isothiocyanate-conjugated anti-MPO (Beckman Coulter); and Pacific blue-conjugated anti-CD15 (Beckman Coulter). Neutrophil granulocytes of patient 1 were sorted on a Becton Dickinson FACSAria with the same antibody combination. The purity of the sorted cells was >98%.
Confocal microscopy
Patient granulocytes were prepared and cytospins were performed as described.14 Cytospins were air dried for 2 hours followed by washing with phosphate-buffered saline. Cells were fixed and permeabilized with ice-cold methanol for 4 minutes at −20°C. Cells were further incubated in blocking buffer (phosphate-buffered saline + 0.5% bovine serum albumin) for 2 hours followed by labeling with an anti-MPO antibody (1:200) for 1 hour, and incubation with Alexa-tagged (488 nm, 647 nm) goat anti-rabbit and goat anti-mouse secondary antibodies (all Life Technologies). 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) was used to stain the nuclei. All the images were captured with a Leica SP5 confocal microscope using SP detection system and LAS AF software (Leica). Imaging was performed on fixed samples at room temperature using a ×63 oil immersion objective with a numerical aperture of 1.4. Images were further processed using ImageJ (U.S. National Institutes of Health).
Relative quantification of MPO messenger RNA (mRNA) by real-time quantitative polymerase chain reaction (qPCR)
RNA was extracted from human whole-blood samples, human peripheral blood granulocytes, and mouse embryonic fibroblasts with the QIAamp RNA Blood Mini Kit (Qiagen). Complementary DNA was generated using the Superscript II reverse transcriptase, nucleoside triphosphates (both Life Technologies), and random hexameres (Roche). qPCR was performed with the Power SYBR Green PCR Master Mix (Applied Biosystems) and 10 nM primers (Qiagen) on an Applied Biosystems 7500 Fast Real-Time PCR System. The human MPO (hMPO) primers (forward, CCGGGATGGTGATCGGTTTT; reverse, CAGATGATCCGGGGCAATGA) were purchased from Microsynth. Porphobilinogen deaminase (forward primer, GGCAATGCGGCTGCAA; reverse primer, GGGTACCCACGCGAATCAC), and mouse glyceraldehyde-3-phosphate dehydrogenase (forward primer, ATTCAACGGCACAGTCAAGG; reverse primer, CTCCACGACATACTCAGCAC) were analyzed as housekeeping genes; SDS software (Applied Biosystems) was used for relative quantification. MPO expression was normalized to the expression of the house-keeping genes as indicated. The data for patient samples is presented as fold change of a reference MF sample with average MPO expression.
Quantification of the CALR mutant allele burden
Genomic DNA was extracted from whole-blood samples or peripheral blood granulocytes with the QIAamp DNA Blood Mini Kit (Qiagen). DNA from cells purified with a fluorescence-activated cell sorter was isolated by phenol chloroform extraction. Fragment analysis of CALR exon 9 was performed as previously reported.11
Immunocytochemistry
Determination of MPO activity by immunocytochemistry was performed as described.15
Expression of hMPO in mouse embryonic fibroblasts
hMPO (NM_000250.1) with an Emerald-tag on the C-terminus (mEmerald-MPO-N18) was obtained from Addgene (#54187). K41 (wild-type [WT]) or K42 (CALR knockout) mouse embryonic fibroblasts were kindly provided by M. Michalak (Edmonton, AB, Canada) and were transfected with 3 μg of MPO-Emerald plasmid.16 Some 24 hours posttransfection, cells were treated with either 10 μM of MG132 (Sigma-Aldrich) or 250 nM of bafilomycin (Sigma-Aldrich) for 6 hours. Cells were then harvested, and analyzed by flow cytometry and by western blot with an anti-MPO antibody (Thermo Fischer Scientific).
Next generation sequencing (NGS) and capillary sequencing
NGS was performed using an Ion torrent personal genome machine sequencer as previously described.17 Primers for target amplification were designed using the ampliseq.com designer (Thermo Fisher Scientific), and target all coding exons of the listed genes (see supplemental Table 1, available on the Blood Web site). Capillary sequencing was performed as previously described, and analysis was performed using Mutation Surveyor (Soft Genetics).17 Primers used to sequence the coding exons of MPO are listed in supplemental Table 2.
Results
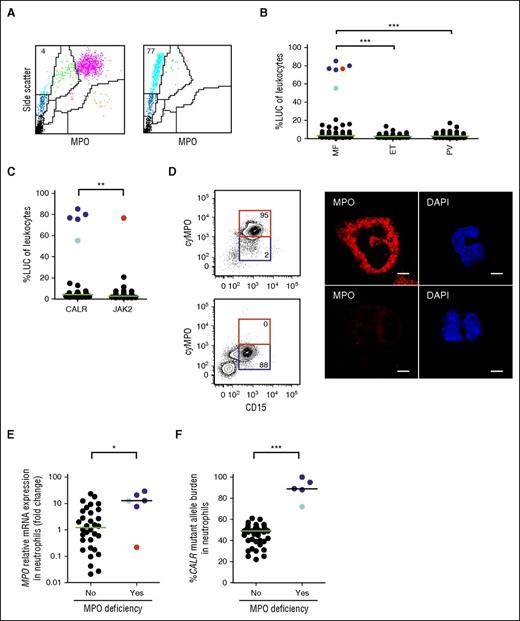
A cohort of 317 MPN patients was screened for MPO deficiency using a flow cytometry-based hematology analyzer that uses light scatter and measures MPO activity to generate a differential leukocyte count (ADVIA 2120).13 Neutrophil granulocytes are identified by the presence of dark precipitates within the cells that are generated by the conversion of H2O2 and 4-chloro-1-naphthol by intracellular MPO. Neutrophil granulocytes devoid of MPO activity lack precipitates and appear as large unstained cells (LUC) (Figure 1A). Complete MPO deficiency (henceforth referred to as MPO deficiency) was defined by the presence of >50% LUC in leukocytes, and confirmed by comparing the number of neutrophil granulocytes determined by ADVIA and the number of neutrophil granulocytes determined by manual count (Table 1). MPO deficiency was observed in 6/81 (7.4%) MF patients but in none of the 142 PV and 94 ET patients studied (Figure 1B). Five of the 6 patients with MPO deficiency (83%) carried a CALR mutation, whereas 1 patient (17%) carried a JAK2-V617F mutation (Figure 1C; Table 1). Two of these patients had a history of serious infectious complications (Table 1). Patient 2 died of pneumonia, had a history of recurrent pulmonary infections, and reactivation of cytomegaly virus. In patient 3, a pulmonary infection with Mycobacterium kansasii and Mycobacterium chimaera had been reported. To quantify MPO protein expression in patients with MPO deficiency, we performed intracellular flow cytometry and confocal microscopy on neutrophil granulocytes from 3 patients with CALR mutations and 1 patient with a JAK2 mutation. In line with the ADVIA data, the MPO protein level was strongly reduced in all 4 patients investigated (Table 1; Figures 1D and 2A-B; supplemental Figures 1 and 2). However, MPO mRNA expression was significantly higher in MF patients with CALR mutations and MPO deficiency as compared with MF patients with normal MPO activity, and 1 patient with a JAK2 mutation and MPO deficiency (Figure 1E). This demonstrates that MPO deficiency in CALR-mutated MF patients results from reduced MPO protein levels and not from decreased MPO mRNA.
Analysis of MPO deficiency in an MPN patient cohort. (A) Peripheral blood ADVIA dot plots from a patient with MF and normal MPO activity (left), and a patient with MF and acquired MPO deficiency (right). LUC (light blue), neutrophil granulocytes (purple), monocytes (green), lymphocytes (dark blue), eosinophil granulocytes (orange), and platelets/lysed red blood cells (black) are shown. The numbers indicate the percentage of LUC in peripheral blood leukocytes. (B) Percentage of LUC in peripheral blood leukocytes from patients with MF (n = 81), ET (n = 94), and PV (n = 142). Patients with a CALR mutation and MPO deficiency (dark blue dots); patient 1 analyzed in more detail in Figure 2 (light blue dot); and patient 6 with a JAK2 mutation and MPO deficiency (red dot) are shown. (C) Percentage of LUC in peripheral blood leukocytes from patients with stratified according to the presence of a CALR (n = 29) or a JAK2 (n = 43) mutation. (D, left) Intracellular detection of MPO protein by flow cytometry in neutrophil granulocytes of a patient with MF and normal MPO activity (top), and a patient with MF and acquired MPO deficiency (bottom). The numbers indicate the percentage of cells in the gate. (D, right) Detection of MPO protein by confocal microscopy in neutrophil granulocytes of a patient with MF and normal MPO activity (top), and a patient with MF and acquired MPO deficiency (bottom). Scale bar, 5 μm. (E) Relative expression of MPO mRNA measured by qPCR and normalized to that of porphobilinogen deaminase in neutrophil granulocytes of patients with MF and normal MPO activity (n = 32), and patients with MPO deficiency (n = 6). (F) Correlation of the presence of MPO deficiency with the mutant allele burden of CALR. The median in each dot plot is represented as a green or black line. *P < .05; **P < .01; ***P < .001 (Mann–Whitney U test). cyMPO, cytoplasmic MPO.
Analysis of MPO deficiency in an MPN patient cohort. (A) Peripheral blood ADVIA dot plots from a patient with MF and normal MPO activity (left), and a patient with MF and acquired MPO deficiency (right). LUC (light blue), neutrophil granulocytes (purple), monocytes (green), lymphocytes (dark blue), eosinophil granulocytes (orange), and platelets/lysed red blood cells (black) are shown. The numbers indicate the percentage of LUC in peripheral blood leukocytes. (B) Percentage of LUC in peripheral blood leukocytes from patients with MF (n = 81), ET (n = 94), and PV (n = 142). Patients with a CALR mutation and MPO deficiency (dark blue dots); patient 1 analyzed in more detail in Figure 2 (light blue dot); and patient 6 with a JAK2 mutation and MPO deficiency (red dot) are shown. (C) Percentage of LUC in peripheral blood leukocytes from patients with stratified according to the presence of a CALR (n = 29) or a JAK2 (n = 43) mutation. (D, left) Intracellular detection of MPO protein by flow cytometry in neutrophil granulocytes of a patient with MF and normal MPO activity (top), and a patient with MF and acquired MPO deficiency (bottom). The numbers indicate the percentage of cells in the gate. (D, right) Detection of MPO protein by confocal microscopy in neutrophil granulocytes of a patient with MF and normal MPO activity (top), and a patient with MF and acquired MPO deficiency (bottom). Scale bar, 5 μm. (E) Relative expression of MPO mRNA measured by qPCR and normalized to that of porphobilinogen deaminase in neutrophil granulocytes of patients with MF and normal MPO activity (n = 32), and patients with MPO deficiency (n = 6). (F) Correlation of the presence of MPO deficiency with the mutant allele burden of CALR. The median in each dot plot is represented as a green or black line. *P < .05; **P < .01; ***P < .001 (Mann–Whitney U test). cyMPO, cytoplasmic MPO.
Characteristics of MF patients with MPO deficiency
| PID . | Diagnosis . | Mutation . | Type . | Mut (%) . | LUC (%) . | NeutroA (G/l) . | NeutroM (G/l) . | MPO (RFI) . | Additional mutations . | Susceptibility to infections . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PET-MF | CALR | 2 | 72 | 55.3 | 1.2 | 4.6 | Low: 3.1; high: 20.8 | None | No |
| 2 | PET-MF | CALR | 2 | 88 | 80 | 0.2 | 2.7 | N/A | KRAS | Yes |
| 3 | PET-MF | CALR | p.K375Rfs*49 | 95 | 85.3 | 0.1 | 15.8 | 6.4 | ASXL1 | Yes |
| 4 | PMF | CALR | p.K375Nfs*55 | 100 | 76.9 | 0.2 | 14.6 | N/A | SF3B1 | No |
| 5 | PMF | CALR | p.K386Nfs*46 | 88.5 | 75.5 | 0.02 | 2.3 | 8.9 | ZRSR2 | No |
| 6 | PMF | JAK2 | V617F | 14 | 76.7 | 0.04 | 8.2 | 5.1 | MPO (2) | No |
| PID . | Diagnosis . | Mutation . | Type . | Mut (%) . | LUC (%) . | NeutroA (G/l) . | NeutroM (G/l) . | MPO (RFI) . | Additional mutations . | Susceptibility to infections . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PET-MF | CALR | 2 | 72 | 55.3 | 1.2 | 4.6 | Low: 3.1; high: 20.8 | None | No |
| 2 | PET-MF | CALR | 2 | 88 | 80 | 0.2 | 2.7 | N/A | KRAS | Yes |
| 3 | PET-MF | CALR | p.K375Rfs*49 | 95 | 85.3 | 0.1 | 15.8 | 6.4 | ASXL1 | Yes |
| 4 | PMF | CALR | p.K375Nfs*55 | 100 | 76.9 | 0.2 | 14.6 | N/A | SF3B1 | No |
| 5 | PMF | CALR | p.K386Nfs*46 | 88.5 | 75.5 | 0.02 | 2.3 | 8.9 | ZRSR2 | No |
| 6 | PMF | JAK2 | V617F | 14 | 76.7 | 0.04 | 8.2 | 5.1 | MPO (2) | No |
Two neutrophil granulocyte populations in patient 1 were distinguished by MPO low and high expression.
LUC (%), percentage of LUCs in leukocytes; Mut (%), ratio of mutated allele to nonmutated allele of CALR and JAK2; N/A, not available; NeutroA, automated neutrophil granulocyte count; NeutroM, manual neutrophil granulocyte count; PET-MF, post-ET MF; PID, patient identification number; PMF, primary MF; RFI, relative fluorescent intensity of MPO to fluorescence minus 1 in neutrophil granulocytes determined by intracellular flow cytometry.
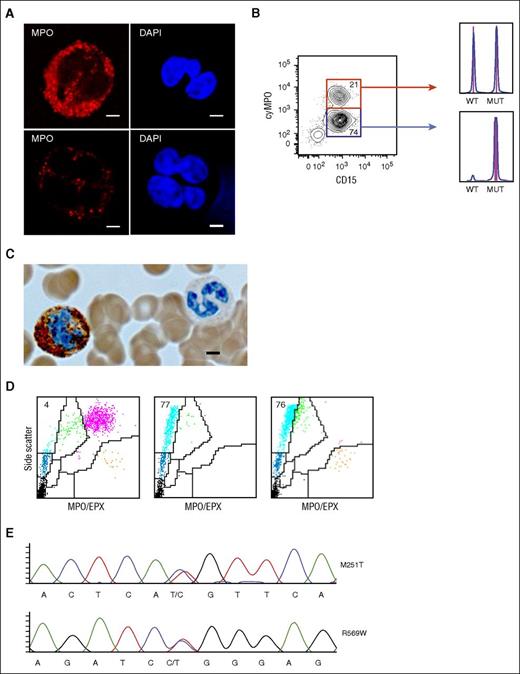
Homozygous CALR mutations lead to MPO and EPX deficiency. Analysis of 2 peripheral blood neutrophil granulocyte populations with distinct MPO protein levels in patient 1. (A) Detection of MPO protein by confocal microscopy in neutrophil granulocytes of patient 1 with normal (top) and reduced (bottom) MPO protein. Scale bar, 5 μm. (B) Purification of the 2 neutrophil granulocyte populations according to the MPO protein level determined by flow cytometry. The numbers indicate the percentage of cells in the gate. DNA was isolated from each fraction and fragment analysis was performed. The respective histograms are shown on the right. Fragment length for CALR WT = 260 bp and for CALR type 2 mutation = 265 bp. MUT, mutated allele. (C) MPO immunocytochemistry of peripheral blood neutrophil granulocytes in patient 1. Brown precipitates represent MPO-positive granules. The ratio of MPO-positive to MPO-negative granulocytes was ∼1:2 (200 neutrophil granulocytes counted manually). Scale bar, 4 μm. (D) Peripheral blood ADVIA dot plots from a patient with MF and normal MPO activity (left), patient 2 with a homozygous CALR mutation and MPO/EPX deficiency (middle), and patient 6 with a JAK2-V617F mutation and MPO deficiency only (right). LUC (light blue), neutrophil granulocytes (purple), monocytes (green), lymphocytes (dark blue), eosinophil granulocytes (orange), and platelets/lysed red blood cells (black) are shown. MPO/EPX, MPO, and EPX-positive cells, respectively. (E) MPO DNA sequences of patient 6. Two mutations were observed in the MPO gene. The base change is denoted below the chromatogram, and the corresponding amino acid change is displayed to the right of the chromatogram. Both mutations have previously been reported to cause hereditary MPO deficiency.19
Homozygous CALR mutations lead to MPO and EPX deficiency. Analysis of 2 peripheral blood neutrophil granulocyte populations with distinct MPO protein levels in patient 1. (A) Detection of MPO protein by confocal microscopy in neutrophil granulocytes of patient 1 with normal (top) and reduced (bottom) MPO protein. Scale bar, 5 μm. (B) Purification of the 2 neutrophil granulocyte populations according to the MPO protein level determined by flow cytometry. The numbers indicate the percentage of cells in the gate. DNA was isolated from each fraction and fragment analysis was performed. The respective histograms are shown on the right. Fragment length for CALR WT = 260 bp and for CALR type 2 mutation = 265 bp. MUT, mutated allele. (C) MPO immunocytochemistry of peripheral blood neutrophil granulocytes in patient 1. Brown precipitates represent MPO-positive granules. The ratio of MPO-positive to MPO-negative granulocytes was ∼1:2 (200 neutrophil granulocytes counted manually). Scale bar, 4 μm. (D) Peripheral blood ADVIA dot plots from a patient with MF and normal MPO activity (left), patient 2 with a homozygous CALR mutation and MPO/EPX deficiency (middle), and patient 6 with a JAK2-V617F mutation and MPO deficiency only (right). LUC (light blue), neutrophil granulocytes (purple), monocytes (green), lymphocytes (dark blue), eosinophil granulocytes (orange), and platelets/lysed red blood cells (black) are shown. MPO/EPX, MPO, and EPX-positive cells, respectively. (E) MPO DNA sequences of patient 6. Two mutations were observed in the MPO gene. The base change is denoted below the chromatogram, and the corresponding amino acid change is displayed to the right of the chromatogram. Both mutations have previously been reported to cause hereditary MPO deficiency.19
Most patients with MF carry a heterozygous CALR mutation, whereas homozygous CALR mutations are rare and most frequently result from uniparental disomy (UPD) of chromosome 19p.11,18 In our cohort, patients with MF and also MPO deficiency carried CALR type 2 (2/5) or non-type 1/2 mutations (3/5), all predicting a novel C-terminal peptide, which lacks the lysine, aspartic acid, glutamic acid, leucine (KDEL) motif, as previously described.10,11 The median mutant allele burden of CALR in patients with MF and MPO deficiency was significantly higher (89%) compared with MF patients with normal MPO activity (49%), consistent with the presence of a dominant subpopulation of cells homozygous for the mutation (Figure 1F and supplemental Figure 3).11,18 Consistent with the data on previously reported patients with homozygous CALR mutations, we also observed a copy neutral loss of heterozygosity of chromosome 19p in all 5 patients investigated (supplemental Figure 4).11 Importantly, we did not find patients with homozygous CALR mutations and normal MPO activity or patients with heterozygous CALR mutations and MPO deficiency in our cohort. The CALR mutant allele burden in neutrophil granulocytes of patient 1 was 72%, and the ADVIA pattern, immunocytochemistry, and confocal microscopy demonstrated the presence of 2 neutrophil granulocyte populations with distinct MPO activity and protein levels (Figures 1F and 2A-C). Considering that in our study MPO deficiency was strongly associated with a homozygous CALR mutated clone, this observation suggested the presence of a homozygous alongside with a heterozygous clone in neutrophil granulocytes. To test this hypothesis, neutrophil granulocytes from patient 1 were purified by fluorescence-activated cell sorting according to the MPO protein level and the mutant allele burden of CALR was determined in the MPO high (normal) and low fractions (Figure 2B). Whereas a heterozygous CALR mutation was detected in neutrophil granulocytes with normal MPO protein, a homozygous CALR mutation was detected in neutrophil granulocytes with low MPO protein, confirming our hypothesis. These observations show that the mutant allele burden of CALR inversely correlates with MPO protein levels and patients with MF with homozygous CALR mutations develop MPO deficiency. Of note, we did not detect reduced CALR protein levels in CD15+ neutrophil granulocytes in 3 patients with homozygous CALR mutations analyzed (supplemental Figure 5). To investigate whether additional recurrent mutations were associated with MPO deficiency in patients with MPN and also CALR mutations, we performed NGS of 22 genes recurrently mutated in MPN (supplemental Table 1).17 Four out of 5 patients carried an additional mutation (Table 1). However, none of these additional mutations were recurrent within these patients. It is therefore unlikely that additional MPN-associated mutations contribute to MPO deficiency or cause UPD of chromosome 19p.
In contrast to patients with homozygous CALR mutations and MPO deficiency, patient 6 with a JAK2 mutation and MPO deficiency had a mutant allele burden of only 14% JAK2-V617F (Table 1). This suggests the presence of a small heterozygous clone and does not correlate with the loss of MPO protein in the vast majority of neutrophil granulocytes in this patient. In addition to MPO activity, the ADVIA cell counter also measures the eosinophilic peroxidase (EPX) activity to determine the eosinophil granulocyte count (Figure 2D). EPX, like MPO is a GP chaperoned by CALR in the ER. All 5 patients with homozygous CALR mutations and complete MPO deficiency showed complete EPX deficiency. However, EPX activity in patient 6 with a JAK2 mutation and MPO deficiency was normal, suggesting a different mechanism for MPO deficiency in this patient. An MPO gene mutation, as described in subjects with inherited MPO deficiency, leads to MPO deficiency but not EPX deficiency and could explain the isolated defect in MPO in patient 6.19 Indeed, sequencing of the MPO gene in patient 6 revealed 2 MPO mutations (MPOM251T and MPOR569W), whereas no MPO mutations were found in patients with CALR mutations and MPO deficiency (Figure 2E and data not shown). Both mutations detected in patient 6 have previously been reported in patients with inherited MPO deficiency.19 MPOR569W results in a maturational arrest of MPO at the apopro-MPO stage and MPOM251T leads to reduced incorporation of heme with markedly decreased enzymatic activity. This demonstrates that MPO deficiency in patient 6 is the result of MPO gene mutations, whereas combined MPO and EPX deficiency in patients with CALR mutations suggests a defect in the CALR-mediated MPO and EPX protein processing.
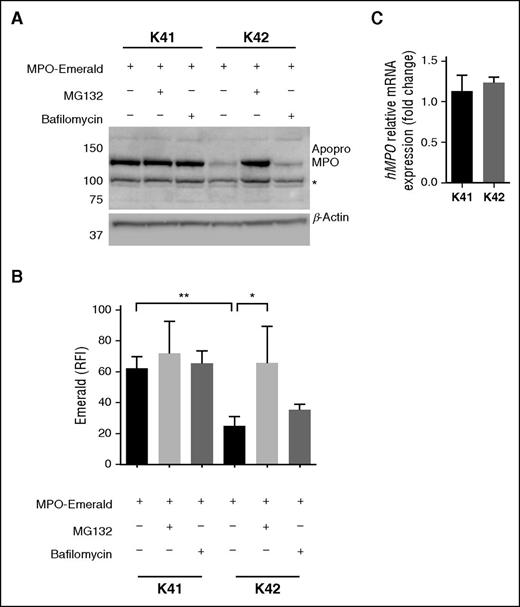
To functionally model homozygous CALR mutations, we investigated hMPO maturation in CALR knockout mouse embryonic fibroblasts (K42) (Figure 3A-B and supplemental Figure 6).16 hMPO was transfected in K42 and WT fibroblasts (K41). In contrast to K41 cells, the expression of the enzymatically inactive MPO precursor protein apopro-MPO was strongly reduced in K42 cells. Both K41 and K42 cells transfected with hMPO showed robust and almost equal expression of hMPO mRNA, again supporting a posttranscriptional defect in MPO production as observed in patients with homozygous CALR mutations (Figure 3C). In patients with an inherited MPO mutation (MPOY173C), apopro-MPO is degraded in the proteasome as a consequence of prolonged association with CALR and CNX, possibly due to abnormal folding of apopro-MPOY173C.6 To further investigate whether the reduced hMPO protein level in K42 cells was the consequence of increased degradation of apopro-MPO, we performed the same experiments in the presence of a proteasome and a lysosome inhibitor. Treatment of K42 cells that were transfected with hMPO with the proteasome inhibitor MG132 resulted in a significant increase of MPO protein, whereas treatment with the lysosome inhibitor bafilomycin had no significant effect (Figure 3A-B). These data show that MPO protein expression in K42 cells relies on the presence of functional CALR and that in the absence of WT CALR, an MPO precursor is produced but then degraded in the proteasome.
Absence of functional CALR leads to MPO deficiency through proteasomal degradation of MPO. (A) Expression of hMPO protein tagged with an Emerald-tag in mouse embryonic fibroblasts (K41) and mouse embryonic fibroblasts derived from a CALR knockout mouse (K42). A proteasome inhibitor (MG132) and a lysosome inhibitor (Bafilomycin) were added to the cultures as indicated. MPO protein levels were assessed by western blot. The expected size for apopro-MPO (with Emerald-tag), and β-Actin are 130 kDa and 42 kDa, respectively. The asterisk indicates an unspecific band also observed in untransfected K41 and K42 cells. (B) RFI of Emerald assessed by flow cytometry in the same conditions as in (A). The experiment was repeated 3 times. (C) Relative expression of hMPO mRNA to that of mouse glyceraldehyde-3-phosphate dehydrogenase in K41 and K42 cells transfected with hMPO. Results are expressed as mean ± standard deviation. *P < .05; **P < .01 (unpaired Student t test).
Absence of functional CALR leads to MPO deficiency through proteasomal degradation of MPO. (A) Expression of hMPO protein tagged with an Emerald-tag in mouse embryonic fibroblasts (K41) and mouse embryonic fibroblasts derived from a CALR knockout mouse (K42). A proteasome inhibitor (MG132) and a lysosome inhibitor (Bafilomycin) were added to the cultures as indicated. MPO protein levels were assessed by western blot. The expected size for apopro-MPO (with Emerald-tag), and β-Actin are 130 kDa and 42 kDa, respectively. The asterisk indicates an unspecific band also observed in untransfected K41 and K42 cells. (B) RFI of Emerald assessed by flow cytometry in the same conditions as in (A). The experiment was repeated 3 times. (C) Relative expression of hMPO mRNA to that of mouse glyceraldehyde-3-phosphate dehydrogenase in K41 and K42 cells transfected with hMPO. Results are expressed as mean ± standard deviation. *P < .05; **P < .01 (unpaired Student t test).
In summary, this data demonstrates that homozygous CALR mutations lead to acquired combined MPO and EPX deficiency by a mechanism involving increased proteasomal degradation.
Discussion
Acquired MPO deficiency was first described in a patient with acute myeloid leukemia and in a patient with myeloid metaplasia (former designation of MF) in 1971 and 1977, respectively.20,21 Four decades later, we demonstrate that acquired MPO deficiency in MPN is the consequence of homozygous mutations in CALR, a chaperone for GPs in the ER. In addition, we show that the biosynthesis of EPX, another GP folded by CALR, is similarly affected.
Consistent with a posttranscriptional defect in the biosynthesis, MPO deficiency did not result from decreased MPO mRNA expression. In fact, MPO mRNA expression was in the upper normal range, pointing toward a compensatory increase in MPO transcription in patients with CALR mutations and MPO deficiency.
Although additional MPN-associated mutations were detected in patients with homozygous CALR mutations, none of them was recurrent within these patients. Furthermore, no other recurrent genetic alterations in the chromosome 19p UPD region described in patients with CALR mutations have been identified.10,11,22 From these findings, together with the dependence of MPO protein maturation on the presence of functional CALR protein in our experiments with CALR knockout mouse embryonic fibroblasts, we infer that homozygous CALR mutations are sufficient to cause acquired MPO deficiency.
Whereas our data shows that homozygous CALR mutations lead to acquired MPO deficiency, MPO deficiency in 1 patient with a JAK2 mutation was the consequence of 2 MPO gene mutations previously reported in subjects with inherited MPO deficiency.19 Therefore, MPO deficiency in this patient is incidental and not a JAK2-V617F–associated phenomenon.
The number of patients with homozygous CALR mutations in our cohort is too small to support an association between a specific mutational subtype of CALR and MPO deficiency. Although we did not observe homozygous CALR type 1 mutations in patients with MPO deficiency, a homozygous CALR type 1 mutation has been described in a patient with an atypical BCR-ABL1–positive MPN. Future investigations will show whether homozygous CALR type 1 mutations can cause acquired MPO deficiency as well.23
CALR mutations do not alter the primary structure of the CALR binding site for GPs but affect the C-terminal domain, which contains a KDEL motif and a Ca2+-binding domain.10,11 Both are essential for the retention and retrieval of CALR in and to the ER.24 Based on our data, we propose that in the presence of homozygous CALR mutants, immature, presumably misfolded MPO precursor proteins undergo proteasomal degradation, which results in MPO deficiency. Although CALR mutants bind and activate MPL through the glycan binding site, a maturation defect of MPL in the presence of CALR mutants has been described.25 Furthermore, truncation of the CALR C-terminal domain reduces the steady state levels of major histocompatibility complex class I heavy chain and tapasin, 2 GPs folded by CALR.26 These data demonstrate that alterations of the CALR C-terminal domain can affect the biosynthesis of GPs. Alterations in the C-terminal domain may also explain the re-localization of CALR mutants in the ER to Golgi intermediate and could result in decreased availability of CALR in the ER to support GP maturation.25 Further studies will need to determine how CALR mutations mitigate the CALR-mediated processing and maturation of GPs in the ER.
The unique association of CALR mutations with MF and ET strongly implicates a role of these mutations in the pathogenesis of MPN. Our study shows that homozygous CALR mutations lead to acquired MPO deficiency in patients with MF and may help to further elucidate the functional consequences of CALR mutations in MPN development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Wildner and Corinne Altenburger for technical assistance.
This study was supported by the Swiss Cancer League (KLS-3298-08-2013) and the Departement Klinische Forschung Research Prize (A.P.A.T.). A.P.A.T. is a recipient of a Cloëtta medical research position. A.K.K.L. is a recipient of a Forschungskredit fellowship from the University of Zurich. Support was also provided by grants from the Swiss National Science Foundation (310000-120724/1 and 32003BB_135712/1) and the Swiss Cancer League (KLS-2950-02-2012) to R.C.S. A.A. is the recipient of an advanced grant of the European Research Council and is supported by grants from the European Union (NEURINOX), the Swiss National Science Foundation, the Clinical Research Priority Programs “Small RNAs” and “Human Hemato-Lymphatic Diseases,” SystemsX.ch, and the Novartis Research Foundation. Funding was also provided from the University of Zurich Research Priority Programs “Human Hemato-Lymphatic Diseases” and “Translational Cancer Research” (M.G.M.).
Authorship
Contribution: A.P.A.T. designed and performed research, analyzed data, and wrote the paper; P.L., A.K.K.L., V.L., and R.M. performed research and analyzed data; R.C.S. and A.A. analyzed data; and M.G.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandre P. A. Theocharides, Division of Hematology, University Hospital Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland; e-mail: alexandre.theocharides@usz.ch.
References
Author notes
P.L. and A.K.K.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal