Key Points
Donor chimerism >20%-30% usually protects against late disease reactivation after day 180 post stem cell transplantation for primary HLH.
Lower levels do not inevitably result in reactivations. The risks of intervention must be weighed against the risk of reactivation.
Abstract
Reduced-intensity conditioning has improved survival after hematopoietic stem cell transplantation (HSCT) for hemophagocytic lymphohistiocytosis (HLH) at the cost of more frequent mixed chimerism. The minimum level of donor chimerism (DC) required to prevent HLH reactivation in humans remains to be determined. In a multicenter retrospective study, 103 patients transplanted for hereditary HLH (2000-2013) and DC permanently or transiently <75% (overall, CD3+, CD56+) were analyzed regarding DC, specific immunologic function, occurrence of systemic reactivations (≥5/8 HLH criteria), partial systemic flares (<5 criteria and HLH-directed treatment), isolated central nervous system reactivations, and management. Recurrence was reported in 18 patients (systemic reactivation n = 11, partial flare n = 3, isolated central nervous system reactivation n = 4). Ten events occurred during profound immune suppression before day 180 (median DC, 10%; range, 1-100%; CD3+ if available, otherwise overall DC), which renders a differentiation between secondary post-HSCT HLH and HLH related to the genetic defect difficult. Eight events occurred between 0.5 and 6.7 years post-HSCT (median DC, 13%; range, 0-30%). In 5 patients, overall and lineage-specific DC were ≤10% for >6 months (median, 5.1; range, 1.1-10 years) without reactivation. A second HSCT was performed in 18 patients (median, DC 4%; range, 0-19%). Death from reactivation occurred in 4 patients (22% of recurrences). Six patients died of transplant complications following a second HSCT (33% of second HSCT). We conclude that a DC >20%-30% is protective against late reactivation. Lower levels do not, however, inescapably result in recurrences. The decision for or against second HSCT must be based on a thorough risk assessment.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening inflammatory syndrome characterized by fever, organomegaly, and pancytopenia. The disease is defined by the HLH 2004 criteria established by the Histiocyte Society,1 containing clinical, laboratory, and cytological parameters. Inflammatory central nervous system (CNS) lesions, in the presence or absence of systemic features, are another characteristic manifestation of the syndrome. The full picture of the condition usually requires aggressive immunosuppressive therapy. However, in patients with hereditary defects predisposing to HLH, such as familial HLH (FHL) 2-5, Griscelli syndrome 2 (GS2), x-linked lymphoproliferative disease (XLP) 1 and 2, and Chédiak Higashi syndrome (CHS), this is not sufficient to provide long-term disease control. The vast majority of patients thus require an allogeneic hematopoietic stem cell transplantation (HSCT) for eventual cure.2
The choice of the conditioning regimen is an important factor contributing to the success of HSCT in HLH. Myeloablative conditioning (MAC) regimens resulted in high transplant-related mortality and morbidity, such as veno-occlusive disease.3-5 The development of reduced-intensity conditioning (RIC) regimens has substantially decreased toxicity and improved survival.6-8 However, RIC is more likely to result in mixed chimerism, which may place the patient at risk of HLH reactivation. The reported frequency of mixed chimerism in HLH patients after MAC is approximately 20% and reaches 50% in a study with high proportion of haploidentical donors.9 RIC results in mixed chimerism in 30% to 75%, depending on donor selection and type of conditioning. Mismatched donors and late (“proximal”) application of alemtuzumab have been identified as factors that increase the occurrence of mixed chimerism.7,10
A careful assessment of the risk of reactivation is important because weaning or withdrawal of immune suppression, donor lymphocyte infusions (DLI), and stem cell boosts as therapeutic measures to stabilize or increase donor chimerism (DC) can all be associated with adverse events, notably graft-versus-host disease (GVHD). The last resort is a second HSCT, which has a number of associated risks. In a mouse model of FHL2 (perforin deficiency), DC in the overall hematopoietic and the CD8+ compartments between 10% to 20% was sufficient to prevent HLH recurrence.11 However, the minimum DC level required to prevent reactivation of HLH in humans remains unknown.
We therefore performed an international retrospective multicenter study to investigate the relationship of DC level and the occurrence of HLH reactivations following HSCT. We also correlated functional immunological assays with the DC level to determine whether these studies can help guiding treatment decisions. Finally, we analyzed the treatment policies in the participating centers when mixed chimerism occurs. The study did not aim at evaluating risk factors for the appearance of mixed chimerism in HLH patients (such as type of conditioning regimen, donor, or GVHD prophylaxis) because this has been addressed in previous studies.6,7,9,10,12
We observed that a DC >20%-30% was protective beyond the period of post-HSCT immune suppression. However, lower DC levels did not necessarily lead to recurrence, even in the long term. In the HLH episodes that occurred during the first 6 months, the distinction was not always clear-cut between HLH related to the genetic defect and secondary HLH related to the HSCT with immunosuppressive treatment and consecutive viral infections.
Methods
Patients
Patients were eligible if they had received an HSCT for hereditary HLH between January 2000 and September 2013 with successful primary engraftment and if they had a DC level (overall and/or CD3+ and/or CD56+) below 75% at least once. Hereditary disease was defined by either a proven genetic defect (FHL2-5, GS2, CHS, XLP1 or 2), an unequivocal defect in flow cytometry assessment (protein expression, CD107a degranulation assay),13,14 clinical course (recurrent disease), or family history. Clinical data were collected, focusing on HLH recurrence after HSCT, potential triggers, interventions against mixed chimerism, and potential triggers that did not result in HLH recurrence (viral infections and reactivations, live and inactivated vaccinations). A hyperinflammatory episode after HSCT was defined as either (1) systemic HLH reactivation if ≥5 HLH criteria1 were fulfilled, (2) partial flare if <5 criteria were fulfilled and HLH-directed treatment was administered, or (3) an isolated CNS-HLH flare if the clinical picture and/or cerebrospinal fluid and/or magnetic resonance imaging scans were suggestive of CNS-HLH and HLH-directed treatment was administered.
Chimerism analysis
Chimerism was assessed according to the standards of the participating center, mostly by variable number tandem repeat analyses, and less frequently by fluorescence in situ hybridization with x and y probes in cases of sex-mismatched donors. In addition to the overall chimerism (mostly whole blood, less frequently bone marrow), lineage-specific chimerism analyses were performed in some patients after enrichment of CD3+ and CD56+ cells. The DC and dates were recorded at the following time points: first occurrence of DC below 75%; the lowest DC level; the DC at last follow-up; and the DC when it first reached the final level (±10%) (ie, the level at last follow-up or death or second HSCT). If applicable, DC was reported at the time of therapeutic interventions (weaning or withdrawal of immune suppression, DLI, stem cell boost, or second HSCT), at recurrence of HLH, and at the time of functional immunological analyses. DC after a second HSCT was not evaluated.
Functional immunological analyses
The results of flow cytometric measurement of protein expression of perforin, signaling lymphocyte activation molecule (SAP; XLP1), and x-linked inhibitor of apoptosis (XIAP [XLP2]), and CD107a degranulation assay and cytotoxicity of natural killer (NK) cells before and after HSCT were recorded, if available. The assays were performed according to the protocols of several laboratories, as previously published.13-16
Statistics and ethics
Statistical analyses were performed with GraphPad Prism by GraphPad Software, La Jolla, CA. To test for differences in distribution, patient characteristics and therapeutic interventions between patient groups were compared with the Mann-Whitney U and Fisher’s exact tests, categorized results of immunological analyses with the Kruskal-Wallis test, and overall and CD3+ DC levels with the Wilcoxon matched pairs test. Correlation between DC and NK cell degranulation capacity was analyzed with Spearman’s model; a linear regression function was calculated. Survival curves were plotted using the Kaplan-Meier method. Differences in overall and event-free survival were assessed with the Mantel-Cox log-rank test; the hazard ratio was calculated. Because of the retrospective nature of the study, multiple testing was not accounted for. The study protocol was approved by the ethics committee of the Hamburg Chamber of Physicians (PV4689). Consent of parents and/or patients was obtained according to local requirements.
Results
Patient cohort
The cohort comprises 103 patients treated at 23 centers, of which 39, 12, and 9 patients were treated in the 3 largest centers. Forty-three of these patients have been previously reported in articles with a focus on HLH and HSCT, but not on mixed chimerism.4,6-8,12,17-19 The genetic defects represent the full spectrum of hereditary forms of HLH, including FHL2-5, GS2, XLP 1 and 2, and CHS (Table 1). Most patients had been diagnosed with HLH and received HSCT in the first 2 years of life; however, age at onset and at HSCT ranged until young adulthood. Three-quarters of patients had received RIC; one-quarter of patients had received MAC.
HLH recurrences
Hyperinflammatory episodes were reported in 18 patients (overview in Table 2; details in supplemental Table 1, available on the Blood Web site). Systemic reactivations occurred in 11 patients and partial flares occurred in 3 patients. Four patients (3 FHL3, 1 CHS) experienced encephalopathy without systemic features of HLH. The diagnosis of isolated CNS-HLH was unequivocal in 1 of these patients (P025). In P066, who had mild CNS symptoms, the diagnosis was based on the clinical picture only. In P001 and P022, who had severe and eventually fatal courses of disease, CNS-HLH was assumed and respective treatment administered, but an unambiguous diagnosis could not be achieved with extensive diagnostic measures, not even at autopsy.
Ten events occurred during the first 180 days (days 27-172), when immune suppression after HSCT is most profound; 5 of these patients were still receiving GVHD prophylaxis. The other 8 events took place up to 6.7 years after HSCT. Infectious triggers, mostly Epstein-Barr virus (EBV) and cytomegalovirus (CMV), were identified at the time of event in 7 patients. Six of these virus-triggered events occurred during the time of profound immune suppression before day 180.
Of the 18 patients with recurrence, 12 had an unrelated (ie, probably wild-type) donor. In the other 6 patients, the genetic status of the related donor was heterozygous carrier (n = 1), wild-type (n = 1), and unknown (n = 4).
Donor chimerism in recurrences
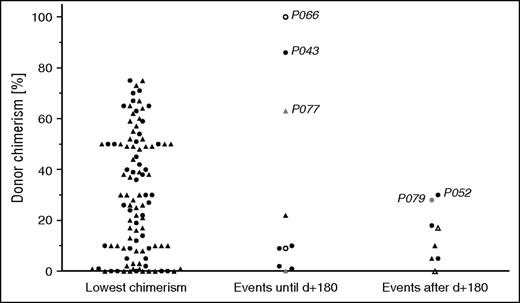
Figure 1 displays the lowest level of DC of each patient (CD3+ if available, otherwise overall DC). In patients in whom overall and CD3+ DC were available, T-cell DC was superior to overall DC. The lowest level in the CD3+ compartment (median, 44%) was significantly higher compared with the lowest overall DC (median, 30%; P = .003; data not shown). The median DC (Figure 1) at the time of the 10 events that occurred during profound immune suppression before day 180 was 10% (CD3+ if available, otherwise overall DC; range, 1-100). Note that even though a DC <75% at least once was one of the inclusion criteria, some events occurred at a time when DC was above that level. In the 8 events after day 180 (all off GVHD prophylaxis), the median DC was 13% (range, 0-30%).
DC in systemic and CNS reactivations and partial flares. The level of DC is depicted, if available, as CD3+ DC, or otherwise as overall DC. Left: Lowest DC level of each patient (CD3+, ▲; overall, ●). Middle and right: Patients with systemic (CD3+, ▲; overall, ●), isolated CNS reactivations (CD3+, △; overall, ○), and partial flares (CD3+, ▲; overall, ●) at the time of the event. Events in the middle column occurred during profound post-HSCT immune suppression before day 180; events in the right column occurred after that day. Refer to the “Results” section for case descriptions of events that occurred at a DC >25%. Patient identifications are indicated in the figure.
DC in systemic and CNS reactivations and partial flares. The level of DC is depicted, if available, as CD3+ DC, or otherwise as overall DC. Left: Lowest DC level of each patient (CD3+, ▲; overall, ●). Middle and right: Patients with systemic (CD3+, ▲; overall, ●), isolated CNS reactivations (CD3+, △; overall, ○), and partial flares (CD3+, ▲; overall, ●) at the time of the event. Events in the middle column occurred during profound post-HSCT immune suppression before day 180; events in the right column occurred after that day. Refer to the “Results” section for case descriptions of events that occurred at a DC >25%. Patient identifications are indicated in the figure.
Events that occurred above a DC of 25% (Figure 1) are described in more detail: P066 displayed unilateral muscle weakness at day 27 post-HSCT when DC was still complete and low CMV viremia was present. Because this patient had had CNS involvement before HSCT, the clinical picture was considered isolated CNS-HLH and treated with dexamethasone, which led to resolution. However, the diagnosis was uncertain because no further diagnostic procedures were performed. In P043, EBV-associated posttransplant lymphoproliferative disease (PTLD) was identified at day 100 and triggered systemic HLH, when overall DC was 86%. Rituximab and methylprednisolone led to remission of PTLD and HLH. P077 had a mild systemic flare with CNS involvement at day 151 while being treated with methylprednisolone, infliximab, and tacrolimus for GVHD. CD3+ DC was 63% (overall DC, 29%; CD56+ DC, 20%); no trigger was identified. The systemic reactivation in P052 occurred off immune suppression at day 188, when the previous overall DC level was 30%; no lineage-specific DC was available. However, a few days later, chimerism measurement showed full autologous reconstitution, indicating that DC had already been rapidly declining at the time of the event. The diagnosis of a partial disease flare in P079, treated with corticosteroids and etoposide, at an overall DC of 28% may in retrospect have to be reconsidered because, except for hemophagocytosis and elevated sCD25, no other HLH criteria were fulfilled (eg, afebrile patient, ferritin 104 µg/L; see supplemental Table 1).
Patients with long-term low-level DC
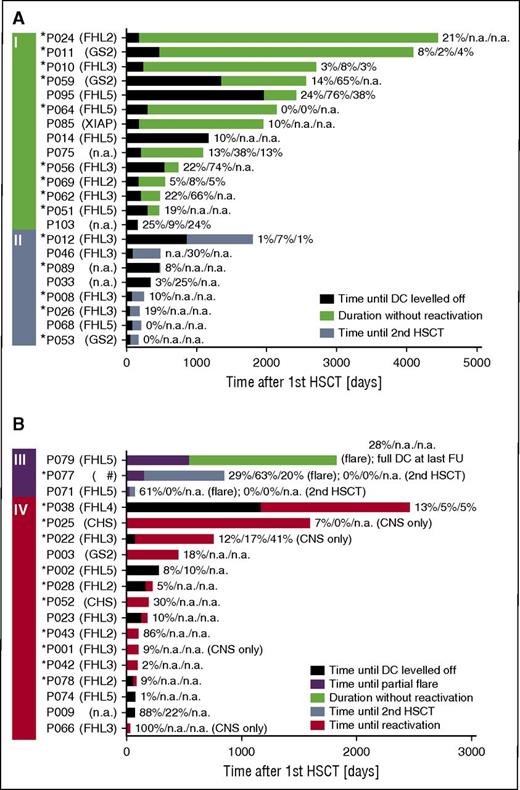
Fourteen patients did not experience recurrences even though they had had a DC <30% for more than 6 months at last follow-up (Figure 2A, group I). Overall, CD3+, and CD56+ DC were <10% for 1.1 to 10 years in 5 patients (P010, P011, P012, P064, P069). In P064, where after 5 years of absent overall and CD3+ DC the donor compartment became detectable again (overall DC, 6%; CD3+ DC, 22%) after a spontaneously resolving episode of fever and cytopenia, the episode may have stimulated the patient’s dormant graft or led to migration of donor cells into the peripheral blood. In 3 other patients (P014, P024, P085) without availability of lineage-specific chimerism, overall DC was 10% to 21% for 3.2 to 11.7 years. Six patients (P046, P056, P059, P062, P075, P095) displayed low overall DC (median, 22%; range, 13-24), but substantially better CD3+ DC (median, 64%; range, 30-76) for a median of 1.2 years (range, 0.6-3.3). Figure 2A-B displays the respective timelines for patients who received a second HSCT without prior recurrence (group II) and for patients with partial flares (group III) and reactivations (group IV).
Timeline after first HSCT. Four groups of patients are included in this figure. (A) Patients who never experienced a reactivation or partial flare, did not have a second HSCT, and had a DC of ≤30% at last follow-up (I). Note that long-term event-free survival was possible in this group despite low DC levels. Patients who did not experience a reactivation or partial flare and received a second HSCT because of low DC (II). (B) Patients with a partial flare (III). Patients with systemic or isolated CNS reactivation (IV). The black part of the bar displays the time from first HSCT until the date when DC leveled off (ie, DC did not vary for >±10% anymore) and the following time until (I) last follow-up (green), (II) second HSCT (blue), (III) partial flare (purple), last follow-up (green), or second HSCT (blue), or (IV) reactivation (red). In some patients, the time when DC leveled off was after an event and is thus not shown. The DC (overall/CD3+/CD56+) at last follow-up (I), at second HSCT (II), at partial flare and last follow-up or second HSCT (III), and reactivation (IV) is provided. In P064, the absent DC for 5 years is shown, even though it became detectable again at last measurement (see the “Results” section for case description). *Patients with early-onset HLH during infancy (≤12 months of age), indicating a severe genetic defect. #A double heterozygous defect was found in this patient, 1 each for FHL3 and 5. CD3, T cell; CD56, NK cell; NA, not available.
Timeline after first HSCT. Four groups of patients are included in this figure. (A) Patients who never experienced a reactivation or partial flare, did not have a second HSCT, and had a DC of ≤30% at last follow-up (I). Note that long-term event-free survival was possible in this group despite low DC levels. Patients who did not experience a reactivation or partial flare and received a second HSCT because of low DC (II). (B) Patients with a partial flare (III). Patients with systemic or isolated CNS reactivation (IV). The black part of the bar displays the time from first HSCT until the date when DC leveled off (ie, DC did not vary for >±10% anymore) and the following time until (I) last follow-up (green), (II) second HSCT (blue), (III) partial flare (purple), last follow-up (green), or second HSCT (blue), or (IV) reactivation (red). In some patients, the time when DC leveled off was after an event and is thus not shown. The DC (overall/CD3+/CD56+) at last follow-up (I), at second HSCT (II), at partial flare and last follow-up or second HSCT (III), and reactivation (IV) is provided. In P064, the absent DC for 5 years is shown, even though it became detectable again at last measurement (see the “Results” section for case description). *Patients with early-onset HLH during infancy (≤12 months of age), indicating a severe genetic defect. #A double heterozygous defect was found in this patient, 1 each for FHL3 and 5. CD3, T cell; CD56, NK cell; NA, not available.
Comparisons between patient groups
There was no evidence that the type or severity of the genetic defect determined the likelihood of recurrence. The distribution of genetic defects in patients with recurrences (group III/IV: FHL2-5, CHS, unknown defects) was similar to patients with low-level DC without recurrence (group I: FHL2-5, XLP2, GS2, unknown defects), see supplemental Table 4A-B. The proportion of patients with early-onset HLH before 12 months of age, as a parameter indicating the severity of the genetic defect, was in the same range in all groups: group I, 9/14 patients (64%); group II, 5/8 patients (63%); and groups III/IV, 11/18 (61%). FHL mutations previously reported as clearly hypomorphic20,21 were found in the same proportion of patients in group I (2/14; 14%), group II (1/8; 13%), and groups III/IV (2/18; 11%). In the latter, however, hypomorphic mutations were only found in patients with partial flares and not with full systemic or CNS reactivations.
To analyze whether T-cell DC is better at predicting outcome than overall DC, we compared both compartments in patients who had reactivations (group IV) and in patients with long-term low-level DC without recurrence (group I), provided availability of both figures. In group I, there was a trend that the lowest CD3+ DC was higher than the lowest overall DC (median, 35% and 14%, respectively; P = .08, n = 10), whereas the CD3+ DC level at the event was similar to overall DC in group IV (median, 10% and 12%, respectively; P = .31, n = 5).
Potential triggers not resulting in HLH
Virus reactivations and infections that did not trigger recurrence of HLH were reported in 63 patients (EBV, 24; adenovirus, 20; CMV, 16; others, 3). The vaccination status post-HSCT was available for 83 patients. Vaccinations had been administered in 51 patients (live and inactivated, n = 38; inactivated alone, n = 13). None resulted in a recurrence of HLH.
Interventions against mixed chimerism
A second HSCT was performed in 18 patients at a median overall DC of 4%, 10 of whom had previously had a recurrence (Table 3; supplemental Table 2). DLI (n = 59) and stem cell boosts (n = 14) were administered at a median overall DC of 45% and 43%, respectively. Weaning or withdrawal of immune suppression was reported for 65 patients at a median overall DC of 85%. For the latter figure, underreporting must be assumed because it was not always possible to determine in retrospect whether a reduction was done routinely or was triggered by a DC result. Interventions were compared between patients with an overall DC always >30% (n = 47) and patients in whom the lowest DC level was ≤30% (n = 56). The 2 groups were similar in the distribution of baseline features (see Table 1). All second HSCTs were performed in the DC ≤30% group, and other interventions were significantly more frequent in this group. Even though interventions were performed sooner after HSCT in the DC ≤30% group, the respective DC levels at intervention were already lower as compared with the DC >30% group (Table 3). This is in keeping with the finding that the decline of DC was steeper in the DC ≤30% group (Figure 3).
Dynamics of loss of donor chimerism. The time interval is shown from first HSCT until overall (A) and CD3+ (B) DC fell below 75% and until overall (C) and CD3+ (D) DC reached the nadir. The intervals were significantly shorter in patients in whom the lowest DC was ≤30% compared with patients who always had a DC >30%. (A) P < .0001; (B) P = .0002; (C) P = .0018; (C) P = .0002.
Dynamics of loss of donor chimerism. The time interval is shown from first HSCT until overall (A) and CD3+ (B) DC fell below 75% and until overall (C) and CD3+ (D) DC reached the nadir. The intervals were significantly shorter in patients in whom the lowest DC was ≤30% compared with patients who always had a DC >30%. (A) P < .0001; (B) P = .0002; (C) P = .0018; (C) P = .0002.
Outcome
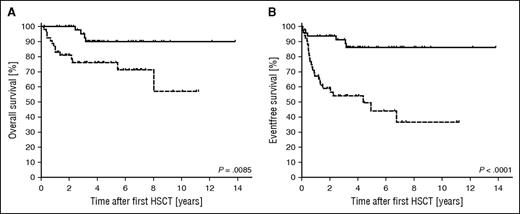
Overall (P = .0085) and event-free (P = .0001) survivals were significantly higher in patients in whom the lowest DC level was always >30% (n = 50; 49%) compared with patients with a lowest DC level ≤30% (n = 53; 51%) (Figure 4). Note that the former group for this analysis includes patients in whom an event occurred at >30%, even if the DC later fell below 30%. The hazard ratios were 3.5 (1.4-8.8) for overall survival and 4.6 (95% confidence interval, 2.3-9.3) for event-free survival. The 18 deaths (Table 4; supplemental Table 3) were related to transplant-related mortality (TRM) of first (n = 6) and second HSCT (n = 6), HLH recurrence (n = 4), secondary malignancy (n = 1), and late sequelae of the initial HLH (n = 1). The outcome of recurrences was fatal in 22% (4/18) and the outcome of second HSCT in 33% (6/18; all TRM). Deaths after second HSCT occurred in similar distribution in patients (3/10; 30%) transplanted from prior recurrence and low DC only (3/8; 38%). Patients with full systemic or CNS reactivations (n = 15) either received a second HSCT (n = 8) or died without second HSCT (n = 5, 4 deaths related to HLH reactivation), except for P043 and P066, in whom HLH had occurred early at a DC of 86% and 100%, respectively (see detailed case description earlier in the text).
Kaplan-Meier estimated overall and event-free survival after first HSCT. Overall survival (A) and event-free survival (B) were significantly better in the group of 50 patients in which the DC was always >30% (solid line) than in the group of 53 patients in which the lowest DC level was ≤30% (dashed line). The respective hazard ratios were 3.5 and 4.6. An event was defined as systemic or isolated CNS reactivation, partial flare, death, or second HSCT.
Kaplan-Meier estimated overall and event-free survival after first HSCT. Overall survival (A) and event-free survival (B) were significantly better in the group of 50 patients in which the DC was always >30% (solid line) than in the group of 53 patients in which the lowest DC level was ≤30% (dashed line). The respective hazard ratios were 3.5 and 4.6. An event was defined as systemic or isolated CNS reactivation, partial flare, death, or second HSCT.
Functional immunological analyses
Flow cytometric analyses of CD107a degranulation or protein expression with the corresponding DC level post-HSCT were available in 34 patients (Figure 5A). DC levels were positively correlated with the proportion of degranulating NK cells (P = .005, Figure 5B). However, differences of DC levels among the 3 groups with absent, reduced, and normal NK cell cytotoxicity were not significant (n = 37, P = .11, Figure 5C). Because perforin, SAP, and XIAP expression was only available in a few patients, correlation with DC levels could not be determined and it was not possible to evaluate whether immunological analyses can predict recurrence of HLH from the limited data at the time of these events (Table 2).
Protein expression and NK cytotoxicity after HSCT. Functional analyses were correlated with the level of DC. Because the depicted assays had been performed with NK cells, CD56+ DC (□) is shown if available, otherwise the overall DC (●) is shown. (A) The results of the NK CD107a degranulation assay without interleukin-2 stimulation (FHL3-5, GS2, and CHS), expression of perforin (FHL2), SAP (XLP1), and XIAP (XLP2) were grouped into absent, reduced, and normal, according to local laboratory standards. (B) The percentage of degranulating (CD107a+) cells and the DC show a significant positive correlation (Spearman P = .005, ρ = .71). (C) NK cytotoxicity results were grouped into absent, reduced, and normal according to local laboratory standards. There was substantial overlap of DC level between the groups (P = .11). PRF, perforin.
Protein expression and NK cytotoxicity after HSCT. Functional analyses were correlated with the level of DC. Because the depicted assays had been performed with NK cells, CD56+ DC (□) is shown if available, otherwise the overall DC (●) is shown. (A) The results of the NK CD107a degranulation assay without interleukin-2 stimulation (FHL3-5, GS2, and CHS), expression of perforin (FHL2), SAP (XLP1), and XIAP (XLP2) were grouped into absent, reduced, and normal, according to local laboratory standards. (B) The percentage of degranulating (CD107a+) cells and the DC show a significant positive correlation (Spearman P = .005, ρ = .71). (C) NK cytotoxicity results were grouped into absent, reduced, and normal according to local laboratory standards. There was substantial overlap of DC level between the groups (P = .11). PRF, perforin.
Discussion
In 18 of 103 patients with mixed chimerism, systemic (n = 11) or CNS (n = 4) reactivations or partial flares (n = 3) were reported. HLH recurred in 15 patients at a DC level ≤30% (CD3+ if available, otherwise overall DC). Three HLH episodes, however, occurred at higher DC levels (63-100%) during the immediate post-HSCT period when patients were severely immunocompromised. Iatrogenic immune suppression is a known predisposing factor to secondary forms of HLH, frequently associated with infection.22 Chemokine and cytokine assays show a hyperinflammatory signature in different post-HSCT complications,23 which may explain a propensity to HLH after HSCT in general. In patients with primary HLH and mixed chimerism, it is thus difficult to determine if a hyperinflammatory episode in this early posttransplant phase must be regarded as HLH related to the underlying genetic or as secondary HLH, which may occur after HSCT for any type of disease. The reported incidence of secondary HLH after HSCT for malignancies in adults ranges from 4% to 17% in the first 9 months.24-27 To account for this uncertainty, we separately analyzed HLH events that occurred during and events that occurred after the phase of most profound immune suppression, with a cutoff at 180 days after HSCT.
HLH episodes before day 180 occurred in 10/18 patients. Viral pathogens could be identified in 6 patients of this group, mostly potent HLH triggers such as EBV and CMV. DC levels were ≤10% in 6 patients, 22% in 1 patient, and 63% to 100% in 3 patients. The latter included a case of EBV-PTLD, a mild partial flare, and a case in which the diagnosis of CNS-HLH must be considered uncertain. We conclude that for patients in this vulnerable phase, low DC is likely to contribute to the development of HLH. However, it is difficult to establish a definite DC threshold that can be considered protective against HLH predominantly related to the genetic defect during this phase.
In the 8 events after day 180, DC levels were ≤10% in 4 patients, 10% to 20% in 2 patients, and 20% to 30% in 2 patients. The diagnosis of a partial flare at a DC of 28% was questionable in retrospect, because only 2 HLH criteria were fulfilled. Reliably diagnosing partial flares is always a challenge, and some degree of uncertainty frequently remains. A reactivation occurred in 1 patient when the DC level was steeply declining from 30% before the event toward full autologous reconstitution shortly after onset, rendering the exact DC at the beginning uncertain. Of note, a weak viral trigger (norovirus) was found in only 1 event. Because none of the pertinent viral triggers is identified in many cases of first presentation of hereditary HLH (except for XLP), this supports the assumption that HLH in this cohort is predominantly attributable to the functional compromise conveyed by low DC levels. We conclude that in patients beyond the phase of profound immune suppression, the likelihood of recurrence of HLH is low, at an approximate DC threshold of 20% to 30%. This figure is similar to a perforin knock-out mouse model, in which a protective level was shown to be at 10% to 20%.11
However, the converse argument that patients will inevitably experience disease recurrence when DC falls below that level does not appear to be true. Fourteen patients without recurrence had low DC ≤30%, and in 5 patients DC was <10%, including CD3+ and CD56+ compartments for up to 10 years. It is noteworthy that in patients with long-term, low-level DC without recurrence, CD3+ DC was higher than overall DC, whereas no difference was seen in patients at reactivation. Given the key role of T cells in HLH pathogenesis,2 this may indicate that higher CD3+ DC may be protective in patients with low overall DC. It is thus advisable to determine CD3+ DC in patients with mixed chimerism.
The affected gene and the severity of the genetic defect did not strongly influence the likelihood of a recurrence. The proportion of HLH onset in infancy was similar in patients with recurrence compared with patients with long-term, low-level DC. Yet, none of the patients with systemic or CNS reactivation had a hypomorphic FHL mutation. Because of limited numbers, we could not evaluate if a heterozygous carrier status of a related donor compared with wild-type donors and if the results of functional immunological analyses had an influence on the likelihood of recurrence. Although a positive correlation of DC and NK cell degranulation in FHL3-5, GS2, and CHS could be shown, DC levels did not consistently predict NK cell cytotoxic capacity. This may be because NK cell cytotoxicity is not only permanently reduced in hereditary defects, but transiently in acquired conditions as well.
The outcome of patients with a DC nadir ≤30% was significantly inferior compared with patients in whom DC never fell below that level. The hazard ratios of overall and event-free survivals were 3.5 and 4.6, respectively, with an event being defined as HLH recurrence, second HSCT, or death. This underscores the need to optimize HSCT to prevent low-level DC (ie, timing, conditioning regimen, donor choice, GVHD, and rejection prophylaxis).6,7,17 The analysis of the current management approaches in patients with declining DC showed that weaning of immune suppression was started at a median DC of 85% and DLI were applied at a median DC of 45%. As expected, interventions were more frequent in the group of patients in which the lowest DC was ≤30%. Because the decline of DC was steeper in the DC ≤30% group, a rapid decline of DC may therefore indicate a high risk of developing profoundly reduced DC and should lead to consideration of interventions. The efficacy of DLI in HLH patients has been demonstrated previously,7,8 but the risk of GVHD has to be taken into account. The reported incidence of acute GVHD grade III-IV after DLI in children with nonmalignant conditions varies between 10% and 20%.7,10
Ten of 18 secondary HSCTs in this study were performed because of HLH recurrence and the other 8 secondary HSCT for low DC level only. HLH recurrences were fatal in 22% of cases. Transplantation-related mortality of second HSCT was 33% and was similarly distributed between patients with and without prior disease recurrence. Although a second HSCT can be considered clearly indicated in cases of late full HLH reactivation, the data do not demonstrate superiority of either preemptive secondary HSCT or watchful waiting in patients with low-level DC without recurrence. For these patients, a tradeoff between risks and benefits has to be made, including consideration of donor choice and overall condition of the patient. A vaccination triggered disease recurrence in none of the patients. Because a true infection with the respective pathogen is more likely to cause harm, we recommend vaccinating patients with stable mixed chimerism according to local standards.
In conclusion, a DC >20% to 30% can be considered protective against HLH reactivation in most patients after day 180. Yet, lower DC levels do not inevitably result in reactivations. The determination of a threshold for patients during profound immune suppression in the first 6 months is more challenging. It is recommended that lineage-specific chimerism be determined because CD3+ DC may differ from overall DC and influence the likelihood of recurrence. When considering a preemptive second HSCT, the risks of reactivation must thus be carefully weighed against the risks of transplant.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following for clinical information: Paul-Gerhardt Schlegel (University Medical Center Würzburg, Germany) and Karl-Walter Sykora (University Medical School, Hannover, Germany). The authors also thank Marcus Tetzlaff and Sandra Standke (University Medical Center Hamburg, Germany) for data management and Udo zur Stadt (University Medical Center Hamburg, Germany) and Ilka Fuchs (Center for Chronic Immunodeficiency, Freiburg, Germany) for laboratory support.
This work was supported by funding from the charitable organizations Fördergemeinschaft Kinderkrebszentrum Hamburg e.V., Histiozytosehilfe e.V. und Charlotte Open (K.L.).
Authorship
Contribution: B.H. collected and analyzed data, designed graphic output, and cowrote the manuscript. R. Marsh, K.R., J.-I.H., M.J., L.F., S.E., G.J., and I.M. contributed to the design of the study and contributed patient data. P.B., R.B., B.B., R. Meisel, A.S., B.W., M.H.A., J.G., G.K., W.W., S.C., B.G., W.H., J.-S.K., P.L., M.G.S., P.V., A.L., and S.A. contributed patient data. K.L. designed the study, contributed patient data, analyzed data, and cowrote the manuscript. All authors approved of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing interests.
Correspondence: Kai Lehmberg, Department of Pediatric Hematology and Oncology, University Medical Center Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany; e-mail: k.lehmberg@uke.de.