Key Points
ATR inhibition is synthetically lethal to TP53- or ATM-defective CLL cells.
ATR targeting induces selective cytotoxicity and chemosensitization in TP53- or ATM-defective CLL cells in vitro and in vivo.
Abstract
TP53 and ataxia telangiectasia mutated (ATM) defects are associated with genomic instability, clonal evolution, and chemoresistance in chronic lymphocytic leukemia (CLL). Currently, therapies capable of providing durable remissions in relapsed/refractory TP53- or ATM-defective CLL are lacking. Ataxia telangiectasia and Rad3-related (ATR) mediates response to replication stress, the absence of which leads to collapse of stalled replication forks into chromatid fragments that require resolution through the ATM/p53 pathway. Here, using AZD6738, a novel ATR kinase inhibitor, we investigated ATR inhibition as a synthetically lethal strategy to target CLL cells with TP53 or ATM defects. Irrespective of TP53 or ATM status, induction of CLL cell proliferation upregulated ATR protein, which then became activated in response to replication stress. In TP53- or ATM-defective CLL cells, inhibition of ATR signaling by AZD6738 led to an accumulation of unrepaired DNA damage, which was carried through into mitosis because of defective cell cycle checkpoints, resulting in cell death by mitotic catastrophe. Consequently, AZD6738 was selectively cytotoxic to both TP53- and ATM-defective CLL cell lines and primary cells. This was confirmed in vivo using primary xenograft models of TP53- or ATM-defective CLL, where treatment with AZD6738 resulted in decreased tumor load and reduction in the proportion of CLL cells with such defects. Moreover, AZD6738 sensitized TP53- or ATM-defective primary CLL cells to chemotherapy and ibrutinib. Our findings suggest that ATR is a promising therapeutic target for TP53- or ATM-defective CLL that warrants clinical investigation.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by biological and clinical heterogeneity.1-4 Two important genes associated with adverse prognosis, TP53 and ataxia telangiectasia mutated (ATM), govern the cellular response to DNA damage, whereby CLL cells, having accumulated sufficient genotoxic damage, are directed to undergo cell cycle arrest or apoptosis.5,6 Disruption of these genes, through gene deletion (ie, 17p for TP53 or 11q for ATM) and/or mutation, confers genomic instability and chemoresistance, leading to an adverse clinical outcome.3,7-16
There is increasing recognition that therapeutic failure and relapse associated with chemotherapy or chemoimmunotherapy can be attributed to selection of DNA damage response (DDR)–defective subclones.17,18 As a result, a paradigm shift has emerged toward the use of DDR-independent therapies, such as B-cell receptor (BCR) signaling inhibitors, in CLL with TP53 defects.19,20 However, although treatment-naïve del(17p) patients exhibited durable response to the BCR signaling inhibitor ibrutinib,21 previously treated individuals with del(17p) or del(11q) continued to demonstrate inferior progression-free and overall survival compared with individuals without these aberrations.19,22 Furthermore, Bruton tyrosine kinase or PLCγ2 mutations,23 clonal evolution,24 early relapses, and Richter transformation25 following ibrutinib treatment have been reported predominantly in patients with del(17p) or del(11q). Many of these patients also acquired complex cytogenetics,23-25 thus underscoring the relevance of genomic instability to therapeutic resistance even for p53-independent treatments. Patients relapsing from BCR signaling inhibitors have limited salvage options and poor clinical outcome, with a median survival of 3.1 months following discontinuation of ibrutinib as reported recently by Jain et al.25 Therefore, therapeutic strategies specifically targeting genomically unstable DDR-defective subclones may be of value for these CLL patients.
Synthetically lethal approaches, in which collaborating pathways that DDR-defective cells depend on for survival are exploited for therapeutic targeting, offer a potential avenue to eradicate CLL cells with DDR defects.26,27 In this respect, we previously demonstrated the utility of poly (ADP-ribose) polymerase inhibition as a targeted therapy for ATM-defective CLL.28 However, synthetically lethal approaches targeting the TP53-defective CLL phenotype have not hitherto been investigated.
Replication stress can arise during physiological cell cycles and is frequently elevated in malignant cells. It occurs when replication fork progression is disrupted and cells continue to cycle despite the presence of unreplicated DNA.29 Ataxia telangiectasia and Rad3-related (ATR) is a serine/threonine protein kinase that regulates replication initiation, preventing aberrant and excessive replication origin firing, which depletes the cellular pool of nucleotides and replication proteins. Inhibition of ATR therefore induces replication stress, manifested by the accumulation of slowed and stalled replication forks with unprotected single-stranded DNA that inevitably breaks. This results in fragmented, partially replicated sister chromatids with free DNA double-stranded ends (DSEs). ATR also mediates the response to replication stress, delaying cell cycle progression, and stabilizing and repairing DNA replication forks.29-31 If ATR is inhibited, maintenance of genome integrity becomes dependent on functional ATM and p53, with ATM being essential for homologous recombination repair (HRR) to restore replication fork topology, and both ATM and p53 for arresting cell cycle progression to permit repair.5,6 ATR, therefore, represents an attractive synthetically lethal target for p53 or ATM deficiency.32,33 Evidence for this synthetically lethal interaction has been previously provided by deletion of ATR in p53-deficient mice34 and by inhibition of ATR in tumor cell lines, which resulted in selective killing of cells harboring p53 or ATM defects.35-37
No study to date has addressed the impact of ATR inhibition on primary tumor samples or xenotransplantation models of hematologic malignancies with DDR defects. Here, we used AZD6738 (AstraZeneca, Alderley Park, United Kingdom), a novel, highly specific, and orally bioavailable ATR kinase inhibitor to examine the effect of ATR inhibition in primary CLL cells, cell lines, and primary CLL xenografts. We present evidence for synthetic lethality and selective cytotoxicity in CLL cells with TP53 or ATM defects, as well as sensitization of these cells to chemotherapeutic agents and ibrutinib.
Materials and methods
Cells and reagents
Details of cell lines and their short hairpin RNA transfections are provided in the supplemental Materials and Methods (available on the Blood Web site). Primary CLL samples (supplemental Table 1; supplemental Figure 1) were obtained from local hospitals with research ethics committee approval. Methods for induction of CLL cell proliferation69 and drugs used are detailed in the supplemental Materials and Methods and supplemental Figure 2.
Western blotting and cytotoxicity assays
Western blotting was carried out as previously described.38 Cytotoxicty was assessed by measurement of propidium iodide uptake and/or CellTiter-Glo luminescence cell viability assay (Promega, Southampton, United Kingdom) as described in the supplemental Materials and Methods.
Primary CLL xenograft models
Animal experiments were conducted in accordance with United Kingdom Home Office regulations. Protocol for the generation of CLL xenografts and treatment regimens are provided in the supplemental Materials and Methods and supplemental Figure 3.
Cell cycle analysis and immunofluorescence microscopy
Cell cycle profiles were obtained using the Accuri C6 flow cytometer (BD Biosciences, Oxford, United Kingdom). Foci and DNA replication tracts were examined using a Nikon E600 Eclipse microscope (Kingston upon Thames, United Kingdom) as described in the supplemental Materials and Methods.
Statistical analysis
Statistical significance was determined using GraphPad Prism (GraphPad Software, San Diego, CA). P ≤ .05 was considered significant.
Results
ATR signaling is active in proliferating CLL cells and is inhibited by AZD6738
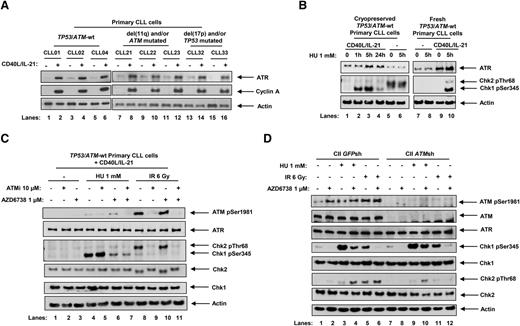
The ATR pathway has been shown to be suppressed in quiescent lymphocytes.39 Consistent with this, we observed that ATR protein expression was low in resting primary CLL cells but induced when cells were stimulated to proliferate by coculturing with CD40 ligand (CD40L)–expressing murine embryonic fibroblasts and interleukin (IL) 21 (CD40L/IL-21). This was irrespective of p53 or ATM status (Figure 1A). We used phosphorylation of checkpoint kinases Chk1 and Chk2, which are downstream targets of ATR and ATM, respectively, as surrogate markers for ATR and ATM pathway activation. Upon treatment with hydroxyurea (HU), which induces replication stress, ATR-dependent Chk1 phosphorylation was observed in proliferating (Figure 1B, lanes 2, 3, and 10) but not quiescent (lanes 6 and 8) CLL cells. The background Chk2 phosphorylation in quiescent cells (lanes 5 and 6) likely reflected cellular stress when cryopreserved samples were preincubated in culture media prior to treatment. This was absent when fresh samples were used without preincubation (lanes 7 and 8). As 24-hour HU treatments (lane 4) appeared to be associated with S-phase checkpoint adaptation, a process in which Chk1 phosphorylation is lost despite persistent replication stress,40 we adopted 5-hour HU treatments for subsequent experiments. The specificity of Chk1 as a marker for ATR activation was evident by minimal Chk1 phosphorylation following ionizing radiation (IR), which generates DNA double-strand breaks and activates the ATM/Chk2 pathway (Figure 1C, lanes 8-11).
ATR signaling is activated in response to replication stress in proliferating primary CLL cells and is inhibited by AZD6738. (A) Stimulation of primary CLL cell proliferation by coculture with CD40L-expressing murine embryonic fibroblasts in the presence of IL-21 (CD40L/IL-21) for 4 days resulted in induction of ATR expression in primary CLL cells irrespective of ATM or TP53 status. Cyclin A expression is a marker of proliferating cells. Actin is the loading control. (B) Cryopreserved or fresh primary CLL cells cultured with or without CD40L/IL-21 (lanes 1-4 and 9 and 10, and lanes 5-8, respectively) were treated with HU. Cryopreserved samples not cocultured with CD40L/IL-21 were resuspended and preincubated in culture media for 24 hours prior to treatment (lanes 5 and 6), whereas fresh cells were treated immediately upon isolation from peripheral blood without preincubation (lanes 7 and 8). Exposure to HU (1 mM), which induces replication stress, led to Chk1 phosphorylation in primary CLL cells cocultured with CD40L/IL-21 (lanes 2, 3, and 10). (C) TP53/ATM wild-type (TP53/ATM-wt) primary CLL cells cocultured with CD40L/IL-21 (C) and CII cells (D), both CII-GFPsh and CII-ATMsh, were treated with AZD6738 (1 μM) and/or the ATM inhibitor KU-55933 (ATMi; 10 μM) for 2 hours, or left untreated, prior to exposure to HU (1 mM) or IR (6 Gy) for a further 5 hours. AZD6738 treatment inhibited ATR signaling as indicated by a reduction in HU-induced Chk1 phosphorylation (panel C, lane 4 vs 6; panel D, lanes 3 vs 4 and 9 vs 10). In ATM-proficient CLL cells, this also led to ATM activation as evidenced by ATM phosphorylation and Chk2 phosphorylation (panel C, lane 4 vs 6; panel D, lane 3 vs 4). In panels B-C, representative blots from experiments on 3 CLL samples are shown.
ATR signaling is activated in response to replication stress in proliferating primary CLL cells and is inhibited by AZD6738. (A) Stimulation of primary CLL cell proliferation by coculture with CD40L-expressing murine embryonic fibroblasts in the presence of IL-21 (CD40L/IL-21) for 4 days resulted in induction of ATR expression in primary CLL cells irrespective of ATM or TP53 status. Cyclin A expression is a marker of proliferating cells. Actin is the loading control. (B) Cryopreserved or fresh primary CLL cells cultured with or without CD40L/IL-21 (lanes 1-4 and 9 and 10, and lanes 5-8, respectively) were treated with HU. Cryopreserved samples not cocultured with CD40L/IL-21 were resuspended and preincubated in culture media for 24 hours prior to treatment (lanes 5 and 6), whereas fresh cells were treated immediately upon isolation from peripheral blood without preincubation (lanes 7 and 8). Exposure to HU (1 mM), which induces replication stress, led to Chk1 phosphorylation in primary CLL cells cocultured with CD40L/IL-21 (lanes 2, 3, and 10). (C) TP53/ATM wild-type (TP53/ATM-wt) primary CLL cells cocultured with CD40L/IL-21 (C) and CII cells (D), both CII-GFPsh and CII-ATMsh, were treated with AZD6738 (1 μM) and/or the ATM inhibitor KU-55933 (ATMi; 10 μM) for 2 hours, or left untreated, prior to exposure to HU (1 mM) or IR (6 Gy) for a further 5 hours. AZD6738 treatment inhibited ATR signaling as indicated by a reduction in HU-induced Chk1 phosphorylation (panel C, lane 4 vs 6; panel D, lanes 3 vs 4 and 9 vs 10). In ATM-proficient CLL cells, this also led to ATM activation as evidenced by ATM phosphorylation and Chk2 phosphorylation (panel C, lane 4 vs 6; panel D, lane 3 vs 4). In panels B-C, representative blots from experiments on 3 CLL samples are shown.
To investigate the effect of AZD6738 on ATR and ATM pathways, we pretreated cycling primary CLL cells and cell lines with 1 μM AZD6738 and/or 10 μM ATM inhibitor KU-55933 (ATMi) for 2 hours prior to HU or IR exposure. AZD6738 treatment led to suppression of ATR signaling as indicated by a reduction in HU-induced Chk1 phosphorylation, independent of ATM status (Figure 1C, lane 4 vs 6; Figure 1D, lanes 3 vs 4, 9 vs 10). Complete abolition of HU-induced Chk1 phosphorylation was evident at AZD6738 doses ≥3 μM (supplemental Figure 4A). The specificity of AZD6738 for ATR inhibition was demonstrated by its lack of effect on IR-induced ATM/Chk2 phosphorylation (Figure 1C, lane 8 vs 10; Figure 1D, lane 5 vs 6). Importantly, in ATM-proficient primary CLL cells and cell line, AZD6738 treatment resulted in ATM activation as evidenced by ATM phosphorylation and HU-induced Chk2 phosphorylation (Figure 1C, lane 4 vs 6; Figure 1D, lane 3 vs 4). This indicated dependence on the ATM pathway in the absence of functional ATR. Because DNA protein kinase (DNA-PK) was reported to have some redundant activity with ATR,41 we assessed the impact of DNA-PK inhibition in primary CLL cells. We detected no change in HU-induced Chk1 phosphorylation upon treatment with DNA-PK inhibitor (supplemental Figure 4B), suggesting that such redundancy is not present in CLL cells.
ATR inhibition is selectively cytotoxic to TP53- or ATM-defective CLL cells in vitro and in vivo
To assess the therapeutic potential of ATR inhibition for DDR-defective CLL, we investigated the cytotoxic effects of AZD6738 on CLL cells. ATM-deficient CII-ATMsh and p53-defective Mec1 cells displayed significantly greater AZD6738 sensitivity (50% effective concentration [EC50], 1.6 μM and 1.1 μM, respectively) following 96-hour treatment compared with ATM/p53-proficient CII-GFPsh cells (EC50, 8.4 μM; Figure 2A). Reintroduction of wild-type TP53 significantly reduced the sensitivity of Mec1 cells to AZD6738 (EC50, 3.2 μM vs 1.0 μM; Figure 2B). The effect was, however, limited because of a moderate level of wild-type p53 expression achieved by transfection relative to expression of the intrinsic mutant TP53 allele (supplemental Figure 5).
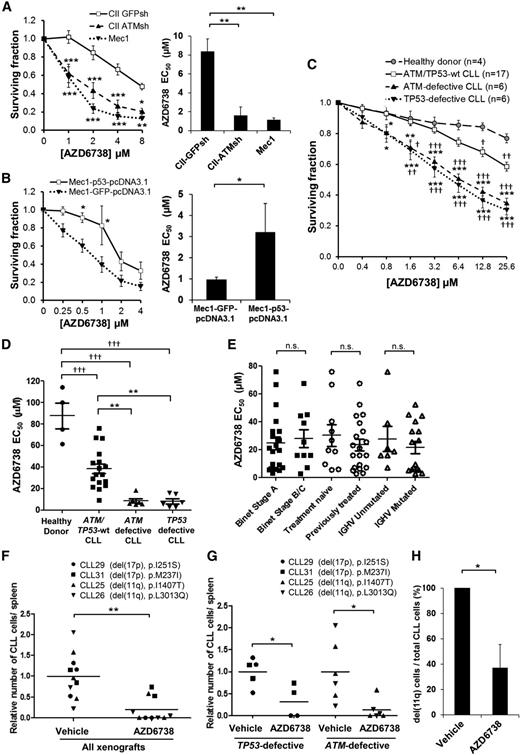
ATR inhibition is selectively cytotoxic to both ATM-defective and TP53-defective CLL cells in vitro and in vivo. (A) CII-GFPsh, CII-ATMsh (ATM-deficient), and Mec1 (p53-defective) cells were treated with AZD6738 for 4 days, and viability was measured using the CellTiter-Glo assay. Surviving fraction is expressed relative to untreated controls. AZD6738 induced significantly greater dose-dependent cytotoxicity with significantly lower AZD6738 EC50 in CII-ATMsh and Mec1 cells compared with CII-GFPsh cells. (B) Mec1 cells transfected with either wild-type TP53 (Mec1-p53-pcDNA3.1) or GFP (Mec1-GFP-pcDNA3.1, as control) were treated with AZD6738 for 4 days, and viability was measured using the CellTiter-Glo assay. Surviving fraction is expressed relative to untreated controls. AZD6738-induced cytotoxicity was reduced with significantly higher AZD6738 EC50 in Mec1-p53-pcDNA3.1 cells compared with Mec1-GFP-pcDNA3.1 cells. (C) Carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled primary CLL cells with or without ATM/TP53 defects and healthy donor peripheral blood mononuclear cells (PBMCs) cocultured with CD40L/IL-21 were treated with AZD6738 for 4 days. Viability was measured by propidium iodide exclusion of the proliferating cell population that was identified by reduction in CFSE fluorescence intensity as shown in supplemental Figure 2. Surviving fraction is expressed relative to untreated controls. AZD6738 induced significantly greater dose-dependent cytotoxicity in ATM/TP53-defective CLL cells than either ATM/TP53 wild-type CLL cells or healthy donor PBMCs. (D) The EC50 of AZD6738 was significantly lower for ATM/TP53-defective primary CLL samples than both ATM/TP53 wild-type samples and healthy donor PBMCs. A list of CLL samples assessed and their respective EC50 values are provided in supplemental Table 1. (E) There was no significant difference in the EC50 of AZD6738 between subgroups of CLL samples stratified according to Binet stage, prior treatment, and IGHV mutational status. (F) Primary CLL samples (CLL29, CLL31, CLL25, CLL26) with biallelic TP53 or ATM defects were engrafted into NOD/Shi-scid/IL-2Rγnull mice and treated with vehicle (n = 11) or AZD6738 (n = 10). Collectively, AZD6738 treatment significantly reduced tumor load compared with vehicle treatment in TP53/ATM-defective xenografts. (G) When analyzed separately, both TP53 and ATM defective xenografts showed significant reduction in tumor load following AZD6738 treatment compared with vehicle-treated controls. (H) Fluorescence in situ hybridization probes for 11q were applied on human CD45+ CD19+ cells collected from the spleens of CLL26 xenografts (harboring del(11q) and L3013Q ATM mutation) treated with AZD6738 (n = 3) or vehicle (n = 3). Two hundred cells were analyzed per mouse. The proportion of CLL cells with del(11q) was significantly reduced following AZD6738 treatment compared with vehicle-treated controls. All data are displayed as mean ± standard error of the mean (SEM). Statistical significance was determined using 2-way analysis of variance (ANOVA) with Bonferroni post hoc analysis (A,B,C,G), 1-way ANOVA (A,D), or Student t test (B,F,H). Statistical significance vs CII-GFPsh (A), Mec1-GFP-pcDNA3.1 (B), ATM/TP53 wild-type samples (*) or healthy donor PBMCs (†) (C,D), or vehicle (F-H) is indicated by *,†P < .05, **,††P < .01, and ***,†††P < .001. Nonsignificant results are denoted by n.s.
ATR inhibition is selectively cytotoxic to both ATM-defective and TP53-defective CLL cells in vitro and in vivo. (A) CII-GFPsh, CII-ATMsh (ATM-deficient), and Mec1 (p53-defective) cells were treated with AZD6738 for 4 days, and viability was measured using the CellTiter-Glo assay. Surviving fraction is expressed relative to untreated controls. AZD6738 induced significantly greater dose-dependent cytotoxicity with significantly lower AZD6738 EC50 in CII-ATMsh and Mec1 cells compared with CII-GFPsh cells. (B) Mec1 cells transfected with either wild-type TP53 (Mec1-p53-pcDNA3.1) or GFP (Mec1-GFP-pcDNA3.1, as control) were treated with AZD6738 for 4 days, and viability was measured using the CellTiter-Glo assay. Surviving fraction is expressed relative to untreated controls. AZD6738-induced cytotoxicity was reduced with significantly higher AZD6738 EC50 in Mec1-p53-pcDNA3.1 cells compared with Mec1-GFP-pcDNA3.1 cells. (C) Carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled primary CLL cells with or without ATM/TP53 defects and healthy donor peripheral blood mononuclear cells (PBMCs) cocultured with CD40L/IL-21 were treated with AZD6738 for 4 days. Viability was measured by propidium iodide exclusion of the proliferating cell population that was identified by reduction in CFSE fluorescence intensity as shown in supplemental Figure 2. Surviving fraction is expressed relative to untreated controls. AZD6738 induced significantly greater dose-dependent cytotoxicity in ATM/TP53-defective CLL cells than either ATM/TP53 wild-type CLL cells or healthy donor PBMCs. (D) The EC50 of AZD6738 was significantly lower for ATM/TP53-defective primary CLL samples than both ATM/TP53 wild-type samples and healthy donor PBMCs. A list of CLL samples assessed and their respective EC50 values are provided in supplemental Table 1. (E) There was no significant difference in the EC50 of AZD6738 between subgroups of CLL samples stratified according to Binet stage, prior treatment, and IGHV mutational status. (F) Primary CLL samples (CLL29, CLL31, CLL25, CLL26) with biallelic TP53 or ATM defects were engrafted into NOD/Shi-scid/IL-2Rγnull mice and treated with vehicle (n = 11) or AZD6738 (n = 10). Collectively, AZD6738 treatment significantly reduced tumor load compared with vehicle treatment in TP53/ATM-defective xenografts. (G) When analyzed separately, both TP53 and ATM defective xenografts showed significant reduction in tumor load following AZD6738 treatment compared with vehicle-treated controls. (H) Fluorescence in situ hybridization probes for 11q were applied on human CD45+ CD19+ cells collected from the spleens of CLL26 xenografts (harboring del(11q) and L3013Q ATM mutation) treated with AZD6738 (n = 3) or vehicle (n = 3). Two hundred cells were analyzed per mouse. The proportion of CLL cells with del(11q) was significantly reduced following AZD6738 treatment compared with vehicle-treated controls. All data are displayed as mean ± standard error of the mean (SEM). Statistical significance was determined using 2-way analysis of variance (ANOVA) with Bonferroni post hoc analysis (A,B,C,G), 1-way ANOVA (A,D), or Student t test (B,F,H). Statistical significance vs CII-GFPsh (A), Mec1-GFP-pcDNA3.1 (B), ATM/TP53 wild-type samples (*) or healthy donor PBMCs (†) (C,D), or vehicle (F-H) is indicated by *,†P < .05, **,††P < .01, and ***,†††P < .001. Nonsignificant results are denoted by n.s.
Following 96-hour AZD6738 treatment, a panel of 29 CD40L/IL-21 cocultured primary CLL samples provided additional evidence for selective cytotoxicity toward CLLs with DDR defects (Figure 2C-D; supplemental Table 1), with a significantly lower EC50 in ATM-defective (8.7 μM; 95% confidence interval [CI], 3.4-13.9 μM; n = 6) and TP53-defective (8.2 μM; 95% CI, 3.5-12.9 μM; n = 6) CLL samples compared with either ATM/TP53 wild-type CLLs (38.3 μM; 95% CI, 28.8-47.8 μM; n = 17) or healthy donor peripheral blood mononuclear cells (PBMCs; 87.6 μM; 95% CI, 50.0-125.3 μM; n = 4). No significant difference in AZD6738 sensitivity was found when these samples were analyzed according to clinical stage, prior treatment, or IGHV mutational status (Figure 2E). As expected, AZD6738 had little cytotoxic effect on cells cultured without CD40L/IL-21, regardless of TP53 or ATM status (supplemental Figure 6). This suggests that AZD6738 specifically targets the proliferating CLL population.
To assess the effectiveness of ATR inhibition in vivo, we used primary CLL xenografts. We engrafted 4 representative primary CLL samples carrying either del(17p) and a TP53 mutation (p.I251S or p.M237I) or del(11q) and an ATM mutation (p.I1407T or p.L3013Q) into NOD/Shi-scid/IL-2Rγnull mice. Following treatment with AZD6738 (n = 10) or vehicle (n = 11) for 2 weeks, tumor load was significantly reduced in AZD6738-treated animals compared with vehicle-treated controls (P ≤ .01; Figure 2F). When analyzed separately according to genotype, both TP53- and ATM-defective AZD6738-treated xenografts showed significant reduction in tumor load (P ≤ .05; Figure 2G). Furthermore, in the CLL26 xenograft where tumor cell recovery allowed monitoring of del(11q) cells, we observed a significant reduction in the percentage of cells with del(11q) (37% vs 100%, P ≤ .05) in AZD6738-treated animals compared with vehicle-treated controls (Figure 2H). Taken together, our in vitro and in vivo data demonstrate efficacy and specificity of AZD6738 for TP53- or ATM-defective CLL.
ATR inhibition induces DNA damage and mitotic catastrophe in TP53- or ATM-defective CLL cells
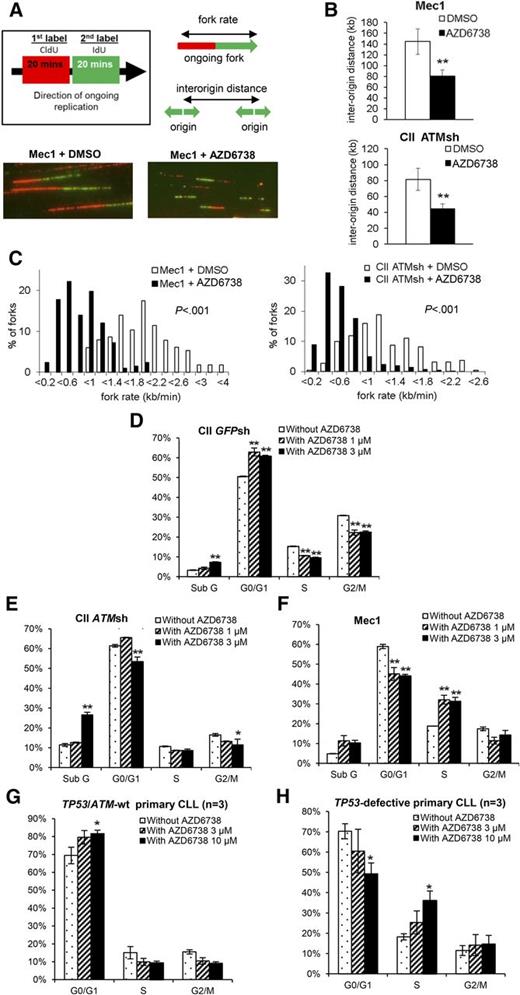
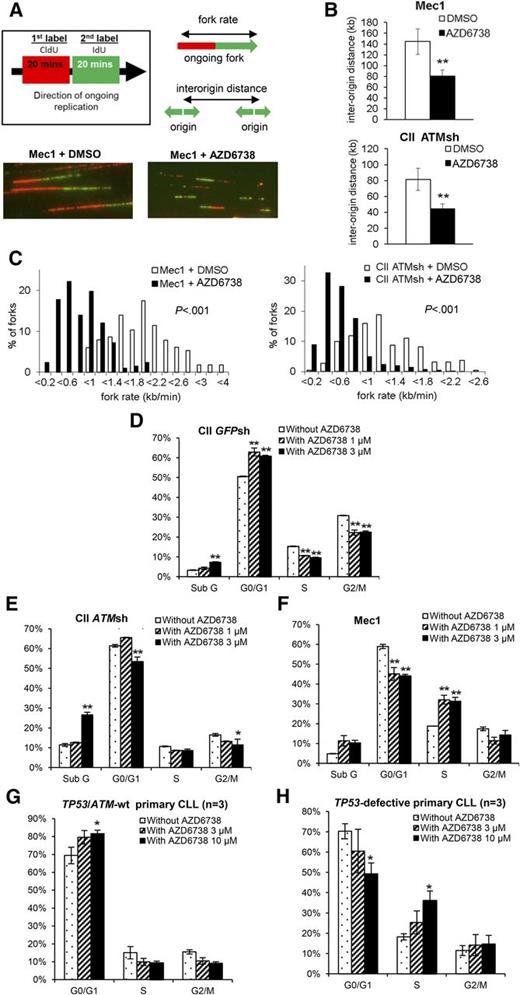
Suppression of aberrant origin firing is an important mechanism whereby ATR protects DNA replication forks from collapse.29-31 We therefore hypothesized that ATR inhibition in CLL cells would result in increased origin firing. To verify this, we first determined the impact of ATR inhibition on DNA replication in CII-ATMsh and Mec1 cells by DNA fiber analysis that visualizes origins of replication and replication fork progression (Figure 3A-C). One hour after treatment with AZD6738, we observed increased replication initiation as indicated by decreased interorigin distance (Figure 3B). We also observed decreased replication fork progression rates (Figure 3C), consistent with fork slowing or stalling and therefore increased replication stress upon ATR inhibition.30,31,42
ATR inhibition leads to increased replication stress and ATM/p53 dependent G1/S cell cycle arrest in CLL cells. (A) AZD6738 (1 μM) treatment of 1 hour led to increased replication stress in Mec1 (p53-defective) and CII-ATMsh (ATM-deficient) cells as demonstrated by DNA fiber analysis. Replicating DNA in cycling cells was sequentially labeled with CldU and IdU for 20 min each, after which DNA fibers were analyzed by immunofluorescence microscopy. Representative images are displayed. (B-C) AZD6738 (1 μM) treatment of 1 hour significantly reduced interorigin distance (B) and fork progression rate (C) in Mec1 and CII-ATMsh cells. (D-F) Cell cycle analyses on CII-GFPsh (D), CII-ATMsh (E), and Mec1 (F) cells were carried out following 24-hour treatment with either AZD6738 (1 μM or 3 μM) or RPMI media. AZD6738 treatment induced G1/S cell cycle arrest in CII-GFPsh cells but not CII-ATMsh or Mec1 cells. (G-H) Cell cycle analyses on TP53/ATM-wt CLL samples (CLL03, CLL06, CLL07) (G) and TP53-defective samples (CLL32, CLL35, CLL36) (H) were carried out following 48-hour treatment with AZD6738 (3 μM or 10 μM) or RPMI media. Analyses in panels G-H were performed on the viable cell population by expressing the G0/G1, S, and G2/M populations as a percentage of the total viable cells. Data are displayed as mean ± SEM of triplicate experiments. Statistical significance was determined using Student t test (B), Mann-Whitney U test (C), or 2-way ANOVA with Bonferroni post hoc analysis (D-H). Statistical significance vs dimethylsulfoxide (DMSO)–treated controls (B) or untreated controls (D-H) is indicated by *P < .05 and **P < .01.
ATR inhibition leads to increased replication stress and ATM/p53 dependent G1/S cell cycle arrest in CLL cells. (A) AZD6738 (1 μM) treatment of 1 hour led to increased replication stress in Mec1 (p53-defective) and CII-ATMsh (ATM-deficient) cells as demonstrated by DNA fiber analysis. Replicating DNA in cycling cells was sequentially labeled with CldU and IdU for 20 min each, after which DNA fibers were analyzed by immunofluorescence microscopy. Representative images are displayed. (B-C) AZD6738 (1 μM) treatment of 1 hour significantly reduced interorigin distance (B) and fork progression rate (C) in Mec1 and CII-ATMsh cells. (D-F) Cell cycle analyses on CII-GFPsh (D), CII-ATMsh (E), and Mec1 (F) cells were carried out following 24-hour treatment with either AZD6738 (1 μM or 3 μM) or RPMI media. AZD6738 treatment induced G1/S cell cycle arrest in CII-GFPsh cells but not CII-ATMsh or Mec1 cells. (G-H) Cell cycle analyses on TP53/ATM-wt CLL samples (CLL03, CLL06, CLL07) (G) and TP53-defective samples (CLL32, CLL35, CLL36) (H) were carried out following 48-hour treatment with AZD6738 (3 μM or 10 μM) or RPMI media. Analyses in panels G-H were performed on the viable cell population by expressing the G0/G1, S, and G2/M populations as a percentage of the total viable cells. Data are displayed as mean ± SEM of triplicate experiments. Statistical significance was determined using Student t test (B), Mann-Whitney U test (C), or 2-way ANOVA with Bonferroni post hoc analysis (D-H). Statistical significance vs dimethylsulfoxide (DMSO)–treated controls (B) or untreated controls (D-H) is indicated by *P < .05 and **P < .01.
We next assessed the impact of ATR inhibition on cell cycle progression. In response to AZD6738, CII-GFPsh cells accumulated in G0/G1 consistent with G1/S cell cycle arrest (Figure 3D; supplemental Figure 7). In contrast, neither G1/S nor G2/M arrest was evident in CII-ATMsh and Mec1 cells despite replication stress (Figure 3E-F; supplemental Figure 7), indicating dependence on ATM and p53 for cell cycle regulation in the absence of ATR. These cell cycle effects were recapitulated in primary CLL cells upon treatment with AZD6738, with G1/S arrest occurring in TP53/ATM wild-type but not TP53-defective samples (Figure 3G-H).
Because ATR inhibition leads to S-phase chromatid fragments,31,32 for which effective resolution requires ATM-mediated HRR and ATM/p53-dependent cell cycle checkpoint activation,5,6 we reasoned that ATR inhibition should produce more fragmented chromatids in ATM/p53-defective CLL cells. Consistent with this, we observed accumulation of markers of DNA damage, γH2AX and 53BP1 foci, in Mec1 and ATMi pretreated CII cells but not in CII cells without ATMi pretreatment, 24 and 48 hours, respectively, following exposure to 1 μM AZD6738 (Figure 4A-E; supplemental Figure 8). Moreover, consistent with loss of both G1/S and G2/M checkpoints in the absence of functional ATR and ATM/p53, Mec1 and ATMi pretreated CII cells carrying unrepaired DNA damage progressed into mitosis, as indicated by dual positivity for γH2AX and the mitotic marker pH3 following 24-hour incubation with 1 μM AZD6738 (Figure 4A-B).
ATR inhibition results in accumulation of DNA damage and mitotic catastrophe in CLL cells with ATM or p53 deficiency. CII cells pretreated for 2 hours with ATMi (10 μM), CII cells without ATMi pretreatment, and Mec1 (p53-defective) cells were exposed to AZD6738. (A-B) Cells treated with AZD6738 (1 μM) for 24 hours were colabeled with anti-γH2AX and anti-pH3 antibodies and analyzed by immunofluorescence microscopy using a ×60 lens. A cell was considered γH2AX positive if >5 γH2AX foci were present. At least 200 mitotic (phosphohistone H3 ser-10 [pH3]–positive) cells were analyzed in each sample. AZD6738 induced γH2AX foci in Mec1 and ATMi pretreated CII cells, but not in CII cells without ATMi pretreatment. (C) The pattern of 53BP1 labeling distinguishes 53BP1 bodies from 53BP1 foci. 53BP1 bodies are characterized by robust blocklike staining and indicate underreplicated DNA. 53BP1 foci are characterized by discrete punctate staining and indicate DNA damage. A cell was considered 53BP1 foci positive if >5 53BP1 foci were present. (D-F) Cells treated with AZD6738 (1 μM) for 48 hours were labeled with anti-53BP1 antibodies, and at least 200 cells were then analyzed in each sample using a ×60 lens. AZD6738 treatment led to an accumulation of 53BP1 foci in Mec1 and ATMi pretreated CII cells and an accumulation of 53BP1 bodies in CII cells without ATMi pretreatment. (G) Colabeling with anti-lamin B and anti-pH3 antibodies allows apoptotic CLL cells to be distinguished from cells undergoing mitotic catastrophe. At least 200 cells were analyzed in each sample. (H) AZD6738 exposure for 72 hours resulted in significantly elevated levels of mitotic catastrophe in Mec1 and ATMi pretreated CII cells. Data are displayed as mean ± SEM of triplicate results from a representative experiment. Statistical significance was determined using Student t test. Statistical significance vs untreated controls is indicated by *P < .05, ** P < .01, and ***P < .001.
ATR inhibition results in accumulation of DNA damage and mitotic catastrophe in CLL cells with ATM or p53 deficiency. CII cells pretreated for 2 hours with ATMi (10 μM), CII cells without ATMi pretreatment, and Mec1 (p53-defective) cells were exposed to AZD6738. (A-B) Cells treated with AZD6738 (1 μM) for 24 hours were colabeled with anti-γH2AX and anti-pH3 antibodies and analyzed by immunofluorescence microscopy using a ×60 lens. A cell was considered γH2AX positive if >5 γH2AX foci were present. At least 200 mitotic (phosphohistone H3 ser-10 [pH3]–positive) cells were analyzed in each sample. AZD6738 induced γH2AX foci in Mec1 and ATMi pretreated CII cells, but not in CII cells without ATMi pretreatment. (C) The pattern of 53BP1 labeling distinguishes 53BP1 bodies from 53BP1 foci. 53BP1 bodies are characterized by robust blocklike staining and indicate underreplicated DNA. 53BP1 foci are characterized by discrete punctate staining and indicate DNA damage. A cell was considered 53BP1 foci positive if >5 53BP1 foci were present. (D-F) Cells treated with AZD6738 (1 μM) for 48 hours were labeled with anti-53BP1 antibodies, and at least 200 cells were then analyzed in each sample using a ×60 lens. AZD6738 treatment led to an accumulation of 53BP1 foci in Mec1 and ATMi pretreated CII cells and an accumulation of 53BP1 bodies in CII cells without ATMi pretreatment. (G) Colabeling with anti-lamin B and anti-pH3 antibodies allows apoptotic CLL cells to be distinguished from cells undergoing mitotic catastrophe. At least 200 cells were analyzed in each sample. (H) AZD6738 exposure for 72 hours resulted in significantly elevated levels of mitotic catastrophe in Mec1 and ATMi pretreated CII cells. Data are displayed as mean ± SEM of triplicate results from a representative experiment. Statistical significance was determined using Student t test. Statistical significance vs untreated controls is indicated by *P < .05, ** P < .01, and ***P < .001.
Replication stress leads to an increase in the amount of underreplicated regions within the genome.29 As the sister chromatids try to separate during anaphase, these catenated regions of DNA result in ultrafine DNA bridges, which often break and are transmitted to daughter cells at the following G1. This can be visualized as 53BP1 nuclear bodies, which serve to protect DNA from endonuclease degradation and facilitate repair.43,44 We observed an accumulation of 53BP1 bodies in ATM/p53-proficient CII cells following 48 hours of AZD6738 treatment (Figure 4F). This was not seen in ATM/p53-defective CLL cells, suggesting that formation of 53BP1 bodies was dependent on the ATM pathway as previously demonstrated.44 Failure to initiate this protective mechanism could precipitate further DNA damage in ATM/p53-defective cells.
We postulated that abolition of cell cycle checkpoints because of the combined loss of functional ATR and ATM/p53 would permit entry into mitosis despite the accumulation of partially replicated chromatid fragments, resulting in mitotic catastrophe. To confirm this, we probed CLL cells for pH3 and lamin B. Cells undergoing normal mitosis stain positive for pH3 but lose lamin B staining. In contrast, cells undergoing mitotic catastrophe do not stain for pH3 but retain lamin B staining, whereas expression of both markers is lost when cells undergo apoptosis (Figure 4G). We found significant levels of mitotic catastrophe 72 hours following 1 μM AZD6738 in Mec1 and ATMi pretreated CII cells, but not in CII cells without ATMi pretreatment (Figure 4H). Because of incomplete DNA replication in the presence of AZD6738, we did not observe accumulation of tetraploid cells (G2/M population) that can accompany mitotic catastrophe (Figure 3E-F,H).45
In ATM/p53-proficient CII cells, we detected low-level apoptotic activity with AZD6738, which is likely a result of p53 activation in response to replication stress (supplemental Figure 9A-B). Mec1 cells also exhibited low levels of apoptotic markers poly (ADP-ribose) polymerase and caspase cleavage (supplemental Figure 9C), but such levels were unlikely to account for their differential sensitivity to ATR inhibition compared with DDR-proficient cells. Indeed, the negligible impact of pan-caspase inhibitor Z-VAD-FMK on the viability of AZD6738-treated Mec1 cells supports this notion (supplemental Figure 9D).
ATR inhibition sensitizes TP53- or ATM-defective CLL cells to chemotherapy and ibrutinib
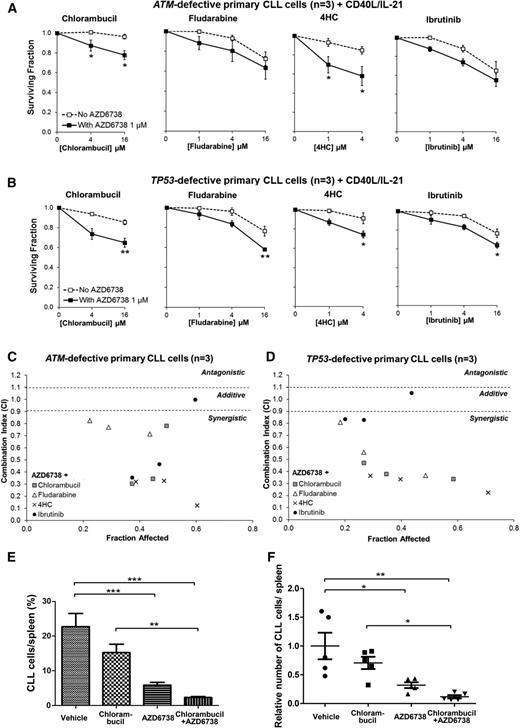
We reasoned that the synthetically lethal effects of ATR inhibition on ATM- or TP53-defective CLL cells could sensitize these chemoresistant cells to chemotherapeutic agents through potentiation of DNA damage. We therefore assessed the impact of combining AZD6738 with chemotherapy in CII-ATMsh and Mec1 cells. Although resistance to chlorambucil, fludarabine, 4-hydroperoxycyclophosphamide, or bendamustine monotherapy was evident even at relatively high doses, we discovered significant sensitization to a range of doses of these agents upon addition of 1 μM AZD6738 (Figure 5; Table 1). Of note, CII-GFPsh cells were sensitive to chemotherapy. However, addition of AZD6738 to chemotherapeutic agents led to further sensitization (supplemental Figure 10; supplemental Table 2).
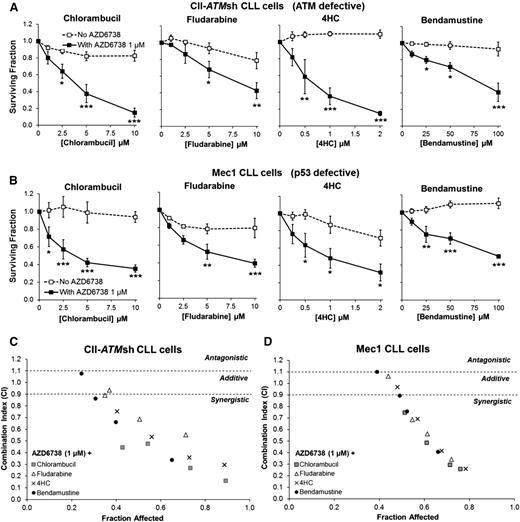
ATR inhibition sensitizes CII-ATMsh and Mec1 CLL cells to cytotoxic chemotherapy. CII-shATM (A) and Mec1 (B) cells were treated with chlorambucil, fludarabine, 4-hydroperoxycyclophosphamide (4HC), or bendamustine with or without coadministration of AZD6738 (1 μM). Viability was assessed 96 hours later by the CellTiter-Glo assay. Surviving fraction is expressed relative to untreated controls for chemotherapy treatment alone (no AZD6738) and relative to 1 μM AZD6738 monotherapy for the cotreated samples. Addition of AZD6738 significantly enhanced sensitivity of CII-shATM and Mec1 cells to cytotoxic chemotherapeutic agents. Data are displayed as mean ± SEM of triplicate experiments. Statistical significance was determined using 2-way ANOVA with Bonferroni post hoc analysis. Statistical significance vs no AZD6738 is indicated by *P < .05, **P < .01, and ***P < .001. (C-D) AZD6738 is synergistic with chlorambucil, fludarabine, 4HC, and bendamustine in CII-shATM (C) and Mec1 (D) cells across a range of effective drug doses. Combination indices (CI) were calculated using the median-effect method. Each point represents the mean CI value obtained from 3 independent experiments plotted against the corresponding affected fraction that is expressed relative to untreated controls. CI <0.9 represents synergism, CI = 0.9-1.1 represents additive effect, and CI >1.1 represents antagonism. The actual values are presented in Table 1.
ATR inhibition sensitizes CII-ATMsh and Mec1 CLL cells to cytotoxic chemotherapy. CII-shATM (A) and Mec1 (B) cells were treated with chlorambucil, fludarabine, 4-hydroperoxycyclophosphamide (4HC), or bendamustine with or without coadministration of AZD6738 (1 μM). Viability was assessed 96 hours later by the CellTiter-Glo assay. Surviving fraction is expressed relative to untreated controls for chemotherapy treatment alone (no AZD6738) and relative to 1 μM AZD6738 monotherapy for the cotreated samples. Addition of AZD6738 significantly enhanced sensitivity of CII-shATM and Mec1 cells to cytotoxic chemotherapeutic agents. Data are displayed as mean ± SEM of triplicate experiments. Statistical significance was determined using 2-way ANOVA with Bonferroni post hoc analysis. Statistical significance vs no AZD6738 is indicated by *P < .05, **P < .01, and ***P < .001. (C-D) AZD6738 is synergistic with chlorambucil, fludarabine, 4HC, and bendamustine in CII-shATM (C) and Mec1 (D) cells across a range of effective drug doses. Combination indices (CI) were calculated using the median-effect method. Each point represents the mean CI value obtained from 3 independent experiments plotted against the corresponding affected fraction that is expressed relative to untreated controls. CI <0.9 represents synergism, CI = 0.9-1.1 represents additive effect, and CI >1.1 represents antagonism. The actual values are presented in Table 1.
We then proceeded to evaluate these findings in DDR-defective primary CLL cells cocultured with CD40L/IL-21. AZD6738 (1 or 3 μM) enhanced sensitivity to (Figure 6A-B) and synergized with (Figure 6C-D; Table 2) chlorambucil, fludarabine, and 4-hydroperoxycyclophosphamide in primary CLL samples with defective ATM or TP53 (n = 6). However, because of prosurvival properties of the coculture system, the sensitization effect (Figure 6A-B) was less profound than in CLL cell lines (Figure 5A-B). Interestingly, in these primary CLL samples, we also found additive to synergistic interaction between AZD6738 and cytotoxic doses of ibrutinib46 (Figure 6C-D; Table 2). We addressed mechanisms behind the AZD6738-ibrutinib interaction. We discovered modest dose-dependent reduction in Erk/Syk phosphorylation with AZD6738 and dose-dependent reduction in HU-induced Chk1 phosphorylation with ibrutinib (supplemental Figure 11), suggesting that off-target effects of AZD6738 and ibrutinib may account for the potentiating effect at higher dose combinations involving 4 μM or 16 μM ibrutinib. We also observed that whereas AZD6738 primarily targeted cycling CLL cells for killing, ibrutinib affected both the cycling and noncycling populations (supplemental Figure 12). This could potentially account for the sensitization seen with 1 μM ibrutinib.
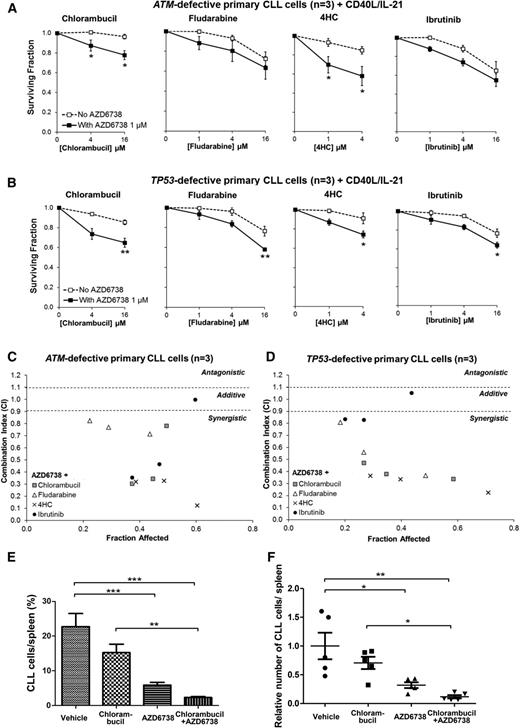
ATR inhibition synergizes with existing therapeutic agents both in ATM-defective and TP53-defective primary CLL cells. CFSE-labeled primary CLL cells with ATM defect (CLL22, CLL23, CLL24) (A) or TP53 defect (CLL29, CLL31, CLL32) (B) cocultured with CD40L/IL-21 were treated with chlorambucil, fludarabine, 4HC, or ibrutinib with or without coadministration of AZD6738 (1 μM). Viability was assessed after 96 hours by propidium iodide exclusion of the proliferating cell population as identified by reduction in CFSE fluorescence intensity. Surviving fraction is expressed relative to untreated controls for chemotherapy treatment alone (no AZD6738) and relative to 1 μM AZD6738 monotherapy for the cotreated samples. Addition of AZD6738 significantly enhanced sensitivity of ATM-defective primary CLL samples to chlorambucil and 4HC, and TP53-defective primary CLL samples to these therapies and also to fludarabine and ibrutinib at ≥1 dose combination. Data are displayed as mean ± SEM. Statistical significance was determined using 2-way ANOVA with Bonferroni post hoc analysis. Statistical significance vs no AZD6738 is indicated by *P < .05, **P < .01, and ***P < .001. (C-D) AZD6738 is synergistic with chlorambucil, fludarabine, 4HC, and ibrutinib in primary CLL samples with ATM (C) or TP53 (D) defect across a range of effective drug doses. CI values were calculated using the median-effect method. Each point represents the mean CI value of 3 samples plotted against the corresponding mean affected fraction that is expressed relative to untreated controls. CI <0.9 represents synergism, CI = 0.9-1.1 represents additive effect, and CI >1.1 represents antagonism. The actual values are presented in Table 2. (E-F) A primary CLL xenograft (CLL25) with a biallelic ATM defect (del(11q) and 1407I>T ATM mutation) was randomized into 4 treatment arms (n = 5 each): AZD6738, chlorambucil, AZD6738-chlorambucil cotreatment, and vehicle. AZD6738 treatment alone or in combination with chlorambucil significantly reduced tumor load relative to vehicle, and the addition of AZD6738 to chlorambucil led to a significantly greater reduction in tumor load relative to chlorambucil monotherapy. The relative number of CLL cells in panel F was normalized to vehicle-treated controls. Data are displayed as mean ± SEM. Statistical significance was determined using 2-way ANOVA with Bonferroni post hoc analysis and is indicated by *P < .05, **P < .01, and ***P < .001.
ATR inhibition synergizes with existing therapeutic agents both in ATM-defective and TP53-defective primary CLL cells. CFSE-labeled primary CLL cells with ATM defect (CLL22, CLL23, CLL24) (A) or TP53 defect (CLL29, CLL31, CLL32) (B) cocultured with CD40L/IL-21 were treated with chlorambucil, fludarabine, 4HC, or ibrutinib with or without coadministration of AZD6738 (1 μM). Viability was assessed after 96 hours by propidium iodide exclusion of the proliferating cell population as identified by reduction in CFSE fluorescence intensity. Surviving fraction is expressed relative to untreated controls for chemotherapy treatment alone (no AZD6738) and relative to 1 μM AZD6738 monotherapy for the cotreated samples. Addition of AZD6738 significantly enhanced sensitivity of ATM-defective primary CLL samples to chlorambucil and 4HC, and TP53-defective primary CLL samples to these therapies and also to fludarabine and ibrutinib at ≥1 dose combination. Data are displayed as mean ± SEM. Statistical significance was determined using 2-way ANOVA with Bonferroni post hoc analysis. Statistical significance vs no AZD6738 is indicated by *P < .05, **P < .01, and ***P < .001. (C-D) AZD6738 is synergistic with chlorambucil, fludarabine, 4HC, and ibrutinib in primary CLL samples with ATM (C) or TP53 (D) defect across a range of effective drug doses. CI values were calculated using the median-effect method. Each point represents the mean CI value of 3 samples plotted against the corresponding mean affected fraction that is expressed relative to untreated controls. CI <0.9 represents synergism, CI = 0.9-1.1 represents additive effect, and CI >1.1 represents antagonism. The actual values are presented in Table 2. (E-F) A primary CLL xenograft (CLL25) with a biallelic ATM defect (del(11q) and 1407I>T ATM mutation) was randomized into 4 treatment arms (n = 5 each): AZD6738, chlorambucil, AZD6738-chlorambucil cotreatment, and vehicle. AZD6738 treatment alone or in combination with chlorambucil significantly reduced tumor load relative to vehicle, and the addition of AZD6738 to chlorambucil led to a significantly greater reduction in tumor load relative to chlorambucil monotherapy. The relative number of CLL cells in panel F was normalized to vehicle-treated controls. Data are displayed as mean ± SEM. Statistical significance was determined using 2-way ANOVA with Bonferroni post hoc analysis and is indicated by *P < .05, **P < .01, and ***P < .001.
Finally, we assessed the AZD6738-chlorambucil combination in a primary xenograft model with del(11q) and ATM mutation p.I407T. Twenty engrafted mice were treated with AZD6738, chlorambucil, the AZD6738-chlorambucil combination, or vehicle (n = 5 in each arm). AZD6738 monotherapy resulted in significant reduction in tumor load. Moreover, combination treatment yielded significantly greater reductions in tumor load than chlorambucil monotherapy (Figure 6E-F), although synergism could not be appropriately assessed from this experiment. Collectively, our results support the combined use of ATR inhibitor with a range of existing therapeutic agents for CLL.
Discussion
In this study, we presented in vitro and in vivo data demonstrating efficacy of ATR inhibition in CLL with TP53 or ATM defects. This report is, to our knowledge, the first demonstration of a therapeutic approach that provides specific targeting of TP53-defective CLL cells.
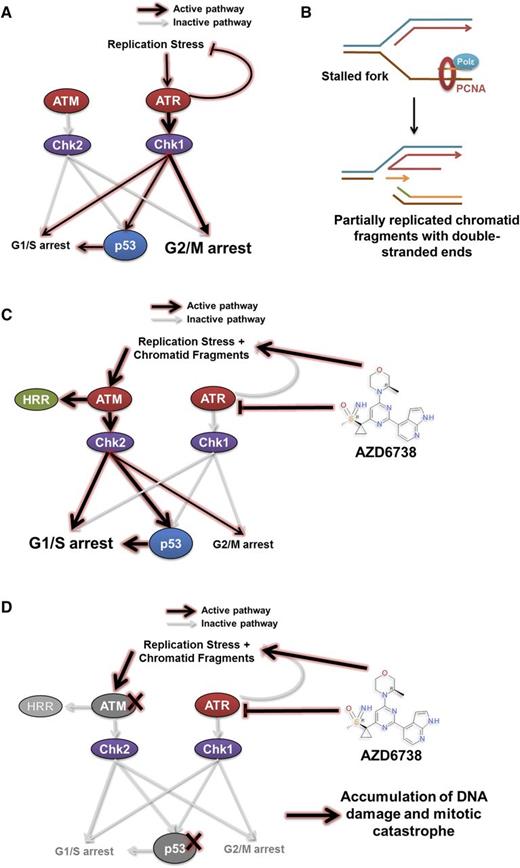
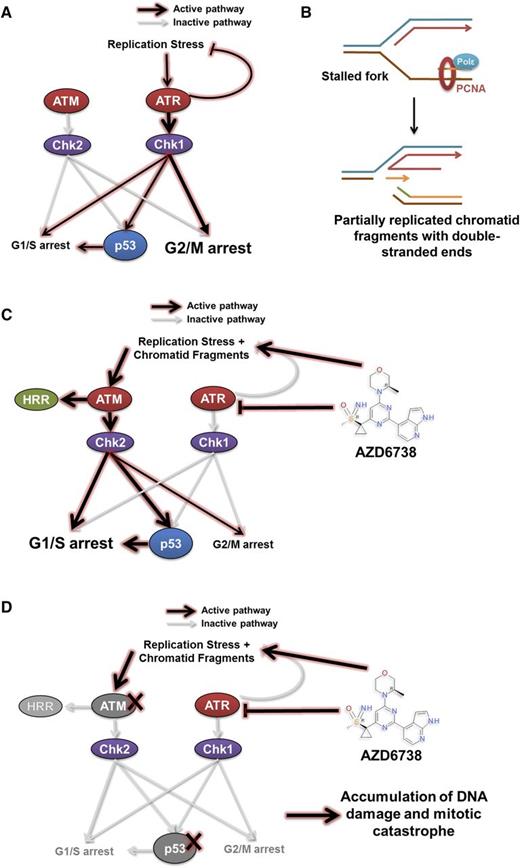
Our findings offer insight into the cytotoxic mechanism underpinning ATR inhibition in CLL, which is consistent with our current understanding of the role of ATR in resolving replication stress (Figure 7A).29-33,47-50 When ATR function is abolished, stalled replication forks with persistent single-stranded DNA are susceptible to nicking and collapse resulting in a partially replicated sister chromatid with a DSE that has become disengaged from the replication template (Figure 7B). Reengagement of the DSE with the replication template requires the HRR stimulatory activity of ATM. Similarly, functional p53 becomes required in the context of ATR inhibition for cell cycle arrest to permit repair of collapsed forks and HRR-directed reengagement of DSEs to restore replication fork geometry (Figure 7C). In the absence of HRR, nonhomologous end joining can ligate 2 independently generated partially replicated sister chromatid DSEs, at the expense of potential sequence deletions and aberrant chromosomal translocations. CLL cells with defective p53 or ATM accumulate collapsed forks and DSEs, whereupon lack of effective cell cycle regulation permits subsequent unrestricted entry into mitosis, resulting in mitotic catastrophe (Figure 7D).
A model for synthetic lethality in CLL cells with ATM or p53 deficiency by inhibition of ATR. ATM and ATR are master regulators of DDR, with ATM being activated in response to DNA double-strand breaks, and ATR in response to replication stress. (A) Activation of the ATR pathway leads to cell cycle arrest mediated primarily through the G2/M checkpoint and repair of stalled replication forks. This leads to the resolution of replication stress. (B-C) Inhibition of ATR by AZD6738 directly induces replication stress by slowing and stalling replication forks. The inability of CLL cells to resolve stalled forks as a result of suppressed ATR signaling leads to collapse of stalled replication forks into fragmented, partially replicated sister chromatids with free DNA DSEs that necessitate repair through the ATM/p53 pathway. This involves cell cycle arrest mediated primarily through the G1/S checkpoint and HRR. (D) In cells with defective ATM or p53, inhibition of ATR by AZD6738 results in an intolerable accumulation of unrepaired DNA damage. This arises from impaired HRR because of defective ATM and/or impaired cell cycle regulation resulting from combined loss of functional ATR and ATM/p53. PCNA, proliferating cell nuclear antigen; Polε, DNA polymerase ε.
A model for synthetic lethality in CLL cells with ATM or p53 deficiency by inhibition of ATR. ATM and ATR are master regulators of DDR, with ATM being activated in response to DNA double-strand breaks, and ATR in response to replication stress. (A) Activation of the ATR pathway leads to cell cycle arrest mediated primarily through the G2/M checkpoint and repair of stalled replication forks. This leads to the resolution of replication stress. (B-C) Inhibition of ATR by AZD6738 directly induces replication stress by slowing and stalling replication forks. The inability of CLL cells to resolve stalled forks as a result of suppressed ATR signaling leads to collapse of stalled replication forks into fragmented, partially replicated sister chromatids with free DNA DSEs that necessitate repair through the ATM/p53 pathway. This involves cell cycle arrest mediated primarily through the G1/S checkpoint and HRR. (D) In cells with defective ATM or p53, inhibition of ATR by AZD6738 results in an intolerable accumulation of unrepaired DNA damage. This arises from impaired HRR because of defective ATM and/or impaired cell cycle regulation resulting from combined loss of functional ATR and ATM/p53. PCNA, proliferating cell nuclear antigen; Polε, DNA polymerase ε.
This mechanistic model accounts for the selective cytotoxicity of ATR inhibition for CLL cells with TP53 or ATM defects. Importantly, ATR inhibition is capable of circumventing the protective effect of the microenvironment, which often hinders effective clearance of genomically unstable, proliferating CLL populations. This is evidenced by the ability of AZD6738 to overcome the prosurvival signals provided by the CD40L/IL-21 coculture system that mimics interaction of CLL cells with T cells in proliferation centers and, in xenograft experiments, by the loss of tumor burden in murine spleens upon treatment with AZD6738.
Interestingly, whereas TP53- or ATM-defective primary CLL samples were uniformly and substantially more sensitive to ATR inhibition than healthy donor PBMCs, TP53/ATM wild-type primary CLL cells displayed variable sensitivity toward ATR inhibition. There are several potential explanations for this finding. Firstly the acquisition of additional, as yet uncharacterized DDR defects could render some CLL patients sensitive to ATR inhibition even in the absence of defective TP53 or ATM. Secondly, such variability in sensitivity could be because of different amounts of endogenous DNA damage in each sample, reflecting varying degrees of genomic instability. ATR inhibition may therefore potentially be most useful in heavily pretreated relapsed/refractory patients, where higher levels of genomic instability could be attributable, in part, to enhanced replication stress. Finally, alternative lengthening of telomeres has been reported in CLL cells51 and could provide an additional mechanism accounting for their sensitivity to ATR inhibition.52 Further work is required to explore these hypotheses and identify markers of sensitivity to ATR inhibition in CLL other than TP53 or ATM defects. With respect to TP53 or ATM defects, the specificity of ATR inhibition for these lesions could allow alteration of the subclonal landscape in favor of less genomically unstable DDR-proficient subclones, which are less susceptible to clonal evolution, thus reducing the likelihood of therapeutic resistance or disease relapse. The specificity of ATR inhibition for TP53-defective CLL cells may possibly also reduce the likelihood of Richter transformation because TP53 defects have been implicated in this process.53,54
Inhibitors of ATR such as AZD6738 could therefore potentially augment current therapies for TP53- or ATM-defective CLL. This is likely to be attributable to potentiation by chemotherapeutic agents of AZD6738-induced replication stress to which TP53- or ATM-defective cells are distinctively susceptible. In CLL, deep remissions attained with chemotherapy or chemoimmunotherapy are translatable into long-term treatment-free survival.55,56 Hence, combination of ATR inhibitor with cytotoxic chemotherapy could provide a realistic salvage option for TP53- or ATM-defective patients relapsing from signaling inhibitors. The synergism of AZD6738 with chlorambucil and bendamustine is particularly attractive given their milder toxicity profiles, making these combinations potentially suitable for older or frailer CLL patients. Although the in vivo toxicity profile and long-term effects of AZD6738 remain to be established in a clinical setting, it is important to note the lack of detrimental effect on normal tissues reported in mice subjected to ATR suppression to 10% of its normal levels.57 Corroborating this report, the AZD6738 dose used in our study to achieve effective tumor load reduction in TP53/ATM-defective xenografts was well tolerated. In addition, the discrepancy between sensitivity of TP53/ATM-defective CLL cells and healthy donor PBMCs to AZD6738 argues for the existence of a substantial therapeutic window making it suitable for clinical use. A potential caveat is that the prolonged use of the ATR inhibitor could allow accumulation of postreplicative damage in nontumor cells as well as ATM/p53 wild-type CLL cells.58 Hence, our current data support the use of ATR inhibition specifically in CLL patients with TP53 or ATM defects, where there is distinct reliance on ATR and sensitivity to ATR inhibition.
Unexpectedly, we found, depending on the dose, additivity or synergism between AZD6738 and ibrutinib in DDR-defective primary CLL cells. The underlying mechanism of the potentiating interaction at higher dose combinations may involve off-target effects of AZD6738 and ibrutinib. At clinically relevant doses (with ≤1 μM ibrutinib), however, this is likely to be accounted for by the limited overlap in the cellular populations that are targeted by these 2 compounds. Should AZD6738 be used in combination with BCR signaling inhibitors, consideration needs to be given to the sequence of treatment because signaling inhibitors, which evict CLL cells from proliferation centers, may render them quiescent and no longer sensitive to ATR inhibition.19 In patients with TP53/ATM-defective CLL, we envisage daily administration of AZD6738 until the attainment of maximum response. Given the relatively high CLL proliferation rate associated with clinically aggressive, relapsed/refractory disease,59 and the potent cytotoxic effect of AZD6738 against proliferating TP53/ATM-defective CLL cells, we anticipate that this could be profound and achievable over weeks to months. Combination with chemotherapy or other targeted therapies would allow simultaneous targeting of both the proliferating and nonproliferating populations, and both DDR proficient and deficient subclones. When combined with ibrutinib, AZD6738 should be initiated first, with ibrutinib added subsequently and dual therapy continued until maximum response is attained.
Finally, TP53 and ATM defects are poor prognostic markers in other hematologic malignancies including mantle cell lymphoma,60 T-prolymphocytic leukemia,61,62 acute myeloid leukemia,63,64 myelodysplastic syndrome,65 multiple myeloma,66,67 and diffuse large B-cell lymphoma.68 Our work on CLL provides a model of how ATR inhibition could selectively target TP53- or ATM-defective cells, and its use in these malignancies could be explored in future studies.
In conclusion, ATR inhibition allows selective targeting of genomically unstable DDR-defective CLL cells, therefore potentially helping to avert clonal evolution, a major cause of treatment refractoriness and disease relapse. The ATR kinase inhibitor AZD6738 is currently being trialed for refractory solid tumors (registered at www.clinicaltrials.gov as #NCT02223923). Our preclinical data provide a basis for similar investigations for refractory TP53/ATM-defective CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Elaine Willmore, Andy Turnell, and Roger Grand for helpful discussions.
This work was supported by a Leukaemia & Lymphoma Research Programme Grant (11045) (T.S.) and Clinical Research Training Fellowship (13059) (M.K.).
Authorship
Contribution: M.K., N.D., and T.S. designed the study; M.K., N.D., A.A., E.S., and E.P. performed experiments; J.B. and A.L. provided AZD6738; G.P., H.P., and P.M. contributed clinical samples; M.K. wrote the manuscript; and M.K., T.S., P.H., G.S., E.P., C.O., and M.T. revised the manuscript.
Conflict-of-interest disclosure: J.B. and A.L. are employees of AstraZeneca Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Tatjana Stankovic, School of Cancer Sciences, University of Birmingham, Institute for Biomedical Research, Vincent Drive, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: t.stankovic@bham.ac.uk.
References
Author notes
M.K. and N.D. contributed equally to this study.

![Figure 4. ATR inhibition results in accumulation of DNA damage and mitotic catastrophe in CLL cells with ATM or p53 deficiency. CII cells pretreated for 2 hours with ATMi (10 μM), CII cells without ATMi pretreatment, and Mec1 (p53-defective) cells were exposed to AZD6738. (A-B) Cells treated with AZD6738 (1 μM) for 24 hours were colabeled with anti-γH2AX and anti-pH3 antibodies and analyzed by immunofluorescence microscopy using a ×60 lens. A cell was considered γH2AX positive if >5 γH2AX foci were present. At least 200 mitotic (phosphohistone H3 ser-10 [pH3]–positive) cells were analyzed in each sample. AZD6738 induced γH2AX foci in Mec1 and ATMi pretreated CII cells, but not in CII cells without ATMi pretreatment. (C) The pattern of 53BP1 labeling distinguishes 53BP1 bodies from 53BP1 foci. 53BP1 bodies are characterized by robust blocklike staining and indicate underreplicated DNA. 53BP1 foci are characterized by discrete punctate staining and indicate DNA damage. A cell was considered 53BP1 foci positive if >5 53BP1 foci were present. (D-F) Cells treated with AZD6738 (1 μM) for 48 hours were labeled with anti-53BP1 antibodies, and at least 200 cells were then analyzed in each sample using a ×60 lens. AZD6738 treatment led to an accumulation of 53BP1 foci in Mec1 and ATMi pretreated CII cells and an accumulation of 53BP1 bodies in CII cells without ATMi pretreatment. (G) Colabeling with anti-lamin B and anti-pH3 antibodies allows apoptotic CLL cells to be distinguished from cells undergoing mitotic catastrophe. At least 200 cells were analyzed in each sample. (H) AZD6738 exposure for 72 hours resulted in significantly elevated levels of mitotic catastrophe in Mec1 and ATMi pretreated CII cells. Data are displayed as mean ± SEM of triplicate results from a representative experiment. Statistical significance was determined using Student t test. Statistical significance vs untreated controls is indicated by *P < .05, ** P < .01, and ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/5/10.1182_blood-2015-05-644872/4/m_582f4.jpeg?Expires=1768259219&Signature=VikzQ-AGkWZaS4LcuNQ69lU~a5HbDJzAFklNK78iyDwu17B0e1w7ezN4xyv2r4t7R5z4Ne4Ojamg-t~VTLheyr3w81mYqpAvNNiR44SiD8ESO1IB0Wxt4Nr27AeluDfyMT5gBlROvyV6ZAMBZxXxkXp1~U9byXN1o2pjy1tHAz214xRIwiRZohUTsXxN--GhIpI3JPE81ZLVCVJB9ASijU5Na755oMQOMMuvlWYosX~UEHccD~lEnkEVvixxPzDfCP8LuGmfunpL~NA2sPTFQAHY7m5N-u8BH~tyMRlj2Gq9dktHxawznhyG8eBsG484CLUSqxbI0XHED7i5XwcJww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 4. ATR inhibition results in accumulation of DNA damage and mitotic catastrophe in CLL cells with ATM or p53 deficiency. CII cells pretreated for 2 hours with ATMi (10 μM), CII cells without ATMi pretreatment, and Mec1 (p53-defective) cells were exposed to AZD6738. (A-B) Cells treated with AZD6738 (1 μM) for 24 hours were colabeled with anti-γH2AX and anti-pH3 antibodies and analyzed by immunofluorescence microscopy using a ×60 lens. A cell was considered γH2AX positive if >5 γH2AX foci were present. At least 200 mitotic (phosphohistone H3 ser-10 [pH3]–positive) cells were analyzed in each sample. AZD6738 induced γH2AX foci in Mec1 and ATMi pretreated CII cells, but not in CII cells without ATMi pretreatment. (C) The pattern of 53BP1 labeling distinguishes 53BP1 bodies from 53BP1 foci. 53BP1 bodies are characterized by robust blocklike staining and indicate underreplicated DNA. 53BP1 foci are characterized by discrete punctate staining and indicate DNA damage. A cell was considered 53BP1 foci positive if >5 53BP1 foci were present. (D-F) Cells treated with AZD6738 (1 μM) for 48 hours were labeled with anti-53BP1 antibodies, and at least 200 cells were then analyzed in each sample using a ×60 lens. AZD6738 treatment led to an accumulation of 53BP1 foci in Mec1 and ATMi pretreated CII cells and an accumulation of 53BP1 bodies in CII cells without ATMi pretreatment. (G) Colabeling with anti-lamin B and anti-pH3 antibodies allows apoptotic CLL cells to be distinguished from cells undergoing mitotic catastrophe. At least 200 cells were analyzed in each sample. (H) AZD6738 exposure for 72 hours resulted in significantly elevated levels of mitotic catastrophe in Mec1 and ATMi pretreated CII cells. Data are displayed as mean ± SEM of triplicate results from a representative experiment. Statistical significance was determined using Student t test. Statistical significance vs untreated controls is indicated by *P < .05, ** P < .01, and ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/5/10.1182_blood-2015-05-644872/4/m_582f4.jpeg?Expires=1768337141&Signature=Xg7bKgCeNq5decZ4DuHrdw3Q7qXwtC7HVnf1E3mlmIRv5PAanZZGE4rSEQvlZGRsMG-LUHdaY7duQJoYX4ldBJWxCVdGQ9J~jt7vvr7~raHt1q2MWPbGHo9CwFyCNeVqdeIZzLRnGRpj97GzAiEyo8lpl6xbIyPufdIA~NXb70U3MZohkfIDjrgU6-EAA9au~dH8IhBgmh4ricllmwPh4lDw7F1khTcvjvajJA3CmrsFGXRTBwr3PetncrpRxESAMzDxnxT~ZuSXGP9Njj0m-5JhiikPXQ3ibLDvapLBgIy~jLbbPyGJ7kjZFwuNDXll4uAE1l6IzAMIWFVDcAbdTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)