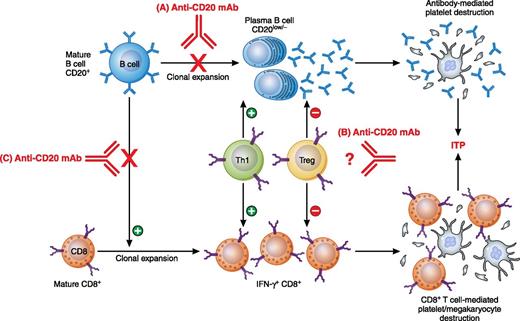
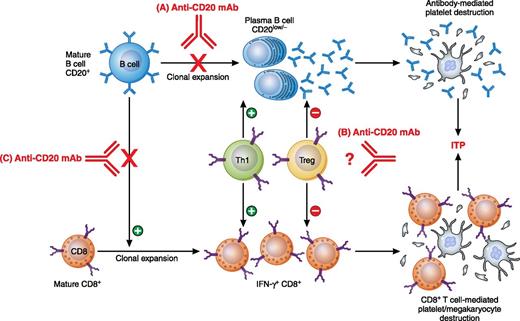
In this issue of Blood, Guo et al used an anti-CD20 monoclonal antibody (mAb) in a murine model of immune thrombocytopenia (ITP) to show for the first time that B-cell depletion therapy inhibits splenic CD8+ T-cell proliferation and rescues T-cell–mediated ITP.1
Previously reported mechanisms of anti-CD20 mAb: (A) depletion of B cells and subsequent decrease in plasma B-cell population and antibody production; (B) downregulation of Th1 and upregulation of Treg populations for unclear reasons. Work by Guo et al revealed a novel mechanism: (C) inhibition of CD8+ T-cell proliferation and amelioration of CD8+ T-cell–mediated pathogenesis. Professional illustration by Patrick Lane, ScEYEnce Studios.
Previously reported mechanisms of anti-CD20 mAb: (A) depletion of B cells and subsequent decrease in plasma B-cell population and antibody production; (B) downregulation of Th1 and upregulation of Treg populations for unclear reasons. Work by Guo et al revealed a novel mechanism: (C) inhibition of CD8+ T-cell proliferation and amelioration of CD8+ T-cell–mediated pathogenesis. Professional illustration by Patrick Lane, ScEYEnce Studios.
B-cell depletion therapy using a chimeric anti-human CD20 mAb, rituximab, is a commonly used treatment of both adults and children with ITP. It is also widely used in several other autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, autoimmune hemolytic anemia, multiple sclerosis, and diabetes. The overall response rate of rituximab in ITP is approximately 60%, with a 20% to 40% treatment-free long-term remission rate.2,3 Rituximab in combination with other therapies, such as corticosteroids or thrombopoietin receptor agonists, have also shown synergistic effects. Thus, rituximab provides a well-tolerated, long-term remission option for salvage treatment of ITP patients.2,3
Since its initial success in ITP management, there has been increased interest in understanding the mechanisms involved in the ameliorating effects of rituximab.4 The initial explanation was that rituximab inhibits antibody-mediated platelet destruction by removal of B cells. However, antiplatelet antibody titers did not necessarily decrease in patients in remission and antiplatelet antibody-negative patients could also respond, suggesting antibody-independent mechanism(s).5 In 2007, Stasi et al reported normalized Th1/Th2 (interferon [IFN]-γ+/interleukin-4+ of CD4+ T cells) and Tc1/Tc2 (IFN-γ+/interleukin-4+ of CD8+ T cells) ratios in the peripheral blood of rituximab responders at 3 and 6 months after treatment but not in patients with active ITP or in rituximab nonresponders, suggesting an immunomodulatory effect of rituximab on both CD4+ and CD8+ T-cell responses.6 Similarly, Nazi et al reported a decreased number of IFN-γ+–producing T cells in patients with ITP upon exogenous antigen stimulation 6 months after rituximab administration.7 Subsequently, further examination of the CD4+ T cells showed restored numbers and function of CD4+ T-regulatory cells (Tregs) after rituximab therapy, which may help explain the restored balance within CD4+ and CD8+ T cells.8 In contrast, spleens from patients who failed rituximab therapy showed that the number of Tregs remained low and the frequency of memory CD8+ T cells increased.9,10 Beyond the changes in numbers and frequencies of different cell populations, our knowledge of how B-cell depletion therapy could regulate CD4+ and CD8+ T-cell responses and restore Tregs in ITP still remains a matter of debate. One limitation is the impossibility of spleen sample acquisition from responder patients for more detailed studies. In addition, although it is well accepted that in patients with ITP, there are both B-cell (antibody)–mediated and CD8+ T-cell–mediated mechanisms, it has been difficult to sort the exact contributions of 1 pathway over the other.
In this issue, a murine model of ITP was used that allows for the study of antibody- and CD8+ T-cell–mediated ITP separately.1 CD61 (GPIIIa) knockout (KO) mice were immunized with wild-type platelets and when their splenocytes were transferred to severe combined immunodeficient (SCID, CD61+) mice, significant thrombocytopenia was induced. By transferring splenocytes that were first B cell–depleted in vitro, CD8+ T-cell–mediated ITP independent of B cells could be induced in the SCID mice. Of interest, administration of a mouse anti-CD20 mAb into the CD61 KO mice before or after platelet immunization significantly inhibited the splenocyte’s ability to induce thrombocytopenia in the SCID mice. It appeared that the CD8+ T-cell defect was associated with decreased CD8+ T-cell proliferation in vitro, which raises the possibility of a novel mechanism of B-cell depletion therapy that limits CD8+ T-cell responses. Although rituximab has been reported to be effective in CD8+ T cell–dominated autoimmune diseases, such as diabetes, this study provides the first mechanistic explanation for the regulation of CD8+ T-cell responses by B cells (see figure).1
Overall, inhibition of CD8+ T-cell proliferation and prevention of the development of ITP by a mouse anti-CD20 mAb suggests a novel role for B cells in maintaining CD8+ T-cell responses. This provides us with an explanation for the therapeutic effect of B-cell depletion therapy for ITP and potentially for other T-cell–mediated autoimmune diseases as well. This may not only improve our understanding of disease pathogenesis, but may also provide helpful information for developing new strategies for ITP management.
Conflict-of-interest disclosure: The author declares no competing financial interests.