In this issue of Blood, Szydlowski et al provide evidence for a major role of the transcription factor subgroup O of forkhead (FOXO1) in mediating the cytotoxic and antiproliferative effects of the spleen tyrosine kinase (SYK) inhibitor R406 in tonic B-cell receptor (BCR) signal-dependent diffuse large B-cell lymphoma (DLBCL).1
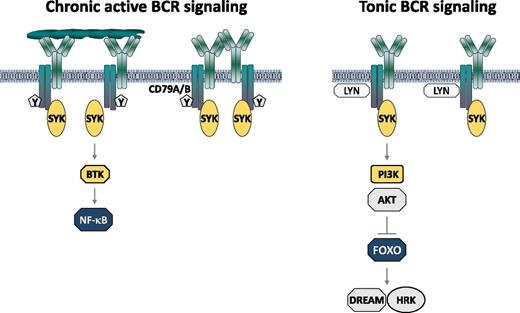
Mechanisms of BCR pathway activation in DLBCL. Mutations in CD79A and CD79B are indicated with Y.
Mechanisms of BCR pathway activation in DLBCL. Mutations in CD79A and CD79B are indicated with Y.
The BCR pathway has emerged as a major therapeutic target in various B-cell malignancies, including DLBCL. In a subset of patients with activated B cell–like (ABC) DLBCL, this pathway is activated by interactions of the tumor immunoglobulins with self-antigens located on the same or surrounding cells (see figure).2 These interactions induce the formation of BCR microclusters, which generate a constitutive signal termed chronic active BCR signal.3 In some patients this signal is further amplified by mutations in the CD79A or CD79B subunits of the BCR, which prevent the recruitment of negative regulators of BCR signaling. A major feature of this signal is the constitutive activation of Bruton tyrosine kinase (BTK) and the NF-κB pathway.3 Importantly, patients with CD79A or CD79B mutations frequently respond to treatment with the BTK inhibitor ibrutinib, providing further evidence that chronic active BCR signaling plays an important role in the pathogenesis of ABC DLBCL.4
In addition to patients with chronic active BCR signaling, a second subset of DLBCL has been identified that is also characterized by activation of the BCR pathway, but in the absence of BCR microclusters or CD79A/CD79B mutations. Activation of the BCR pathway in these cases is presumed to be antigen-independent and to occur as a consequence of a tonic BCR signal that is enhanced by genetic alterations of BCR signaling pathway components, such as SYK amplification or phosphatase and tensin homolog (PTEN) deletion.5 These lymphomas typically belong to the germinal center B cell–like (GCB) DLBCL subset and are characterized by low baseline NF-κB activity but increased activity of the PI3K/AKT pathway. Importantly, inhibition of BCR signaling in these lymphomas by the SYK inhibitor R406 induces apoptosis, which is primarily mediated by induction of the proapoptotic BCL2 family member, HRK.5
In this issue of Blood, Szydlowski and colleagues further characterize the components of the proapoptotic pathway that is repressed by the tonic BCR signal.1 They show that inhibition of the tonic BCR signal results in increased activation of FOXO1 and increased expression of its target genes, including the proapoptotic BCL2 family member, BCL2L11, and the cell-cycle inhibitor p27. Importantly, they also show that depletion of FOXO1 by RNA interference abrogates R406-induced cytotoxicity in DLBCL cells with tonic BCR signaling, but not in cells with chronic-active BCR signaling. The reduced sensitivity to R406-induced apoptosis in cells with depleted FOXO1 is associated with reduced induction of HRK, further suggesting that FOXO1 is the critical mediator of the proapoptotic pathway that is repressed by the tonic BCR signal. Finally, Szydlowski et al investigate the mechanism through which FOXO1 regulates HRK expression and show that it involves inactivation of the HRK transcriptional repressor DREAM through a caspase-dependent process that is activated by transcriptional targets of FOXO1.
These findings are important because they provide further support for clinical testing of BCR signaling inhibitors not only in DLBCL with chronic active BCR signaling, but also in DLBCL that is dependent on tonic BCR signaling. A crucial issue for future studies will be to determine what type of BCR signaling inhibitor would be most appropriate for patients belonging to each of the 2 DLBCL subsets with an activated BCR pathway. Based on the results of this study and the study of Chen et al,5 both SYK and PI3K inhibitors would be expected to be active in BCR-dependent DLBCL, independently of the mechanism that activates the BCR pathway. Although clinical data are scarce, the SYK inhibitor fostamatinib, which is the prodrug of R406, has already shown clinical activity in patients with DLBCL, with 22% (5/23) of the treated patients responding to treatment.6 A similar response rate was observed in the phase 1/2 clinical trial with ibrutinib in patients with relapsed/refractory DLBCL, with 25% (20/80) of patients responding to treatment.4 Interestingly, however, no patient with DLBCL responded to treatment with the selective PI3Kδ inhibitor idelalisib in the phase 1 study of relapsed/refractory non-Hodgkin lymphoma.7 The lack of responses in that study is probably a result of the small number of enrolled DLBCL patients (n = 9), but may also indicate that targeting PI3Kδ is insufficient to completely block the tonic BCR signal. This possibility needs to be considered in view of findings in murine models suggesting that the tonic BCR signal can be mediated by either PI3Kα or PI3Kδ, whereas antigen-dependent signaling is mediated only by PI3Kδ.8,9 Moreover, clinical responses have been observed in DLBCL patients with the dual PI3K-δ/PI3K-α inhibitor BAY 80–6946,10 indicating that both PI3K isoforms could be involved in transducing BCR signals in DLBCL.
Another important question that still remains unresolved is how to identify DLBCL patients who are most likely to respond to treatment with BCR signaling inhibitors. The use of the ABC DLBCL gene-expression signature has been proposed as a biomarker to enrich for patients with chronic active BCR signaling that are more likely to be sensitive to ibrutinib treatment, based on the higher response rate in ABC than GCB DLBCL (37% vs 5%, respectively).4 In the study by Szydlowski et al, expression of FOXO1, which was absent in 20% of the tumors, was proposed as a potential biomarker to identify patients who should be resistant to SYK or PI3K/AKT inhibitors. Although these biomarkers are undoubtedly valuable, the majority of patients that they would select would still be expected to be unresponsive to treatment. Therefore, further efforts to identify biomarkers for tonic and chronic BCR pathway activation will be essential for the development of personalized therapies with BCR signaling inhibitors in DLBCL.
Conflict-of-interest disclosure: The author declares no competing financial interests.