In this issue of Blood, Garcia-Montero et al1 reported that in nearly 30% of patients with indolent systemic mastocytosis (SM), the characteristic c-kit mutation D816V,2 is not restricted to mast cells, but may also be found in bone marrow (BM)–derived mesenchymal stem cells (MSCs), also known as BM stromal cells (BMSCs).3 Thus, the notion that mastocytosis is a disease driven exclusively by mutations in blood cells, especially mast cells, has now been challenged.
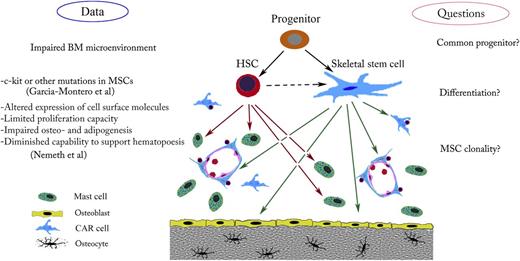
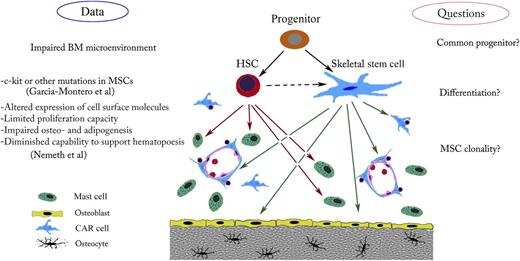
Possible cellular targets and effects of gain-of-function c-kit mutations. In the BM microenvironment, a mutation in a common hematopoietic stem cell (HSC)/skeletal stem cell progenitor could affect both mast cells and the hematopoietic supportive stroma. Such a mutation might explain defects in bone, cartilage, or adipose tissue. Mutation in these cells, which arise from the common progenitor, might also disturb BM homeostasis.
Possible cellular targets and effects of gain-of-function c-kit mutations. In the BM microenvironment, a mutation in a common hematopoietic stem cell (HSC)/skeletal stem cell progenitor could affect both mast cells and the hematopoietic supportive stroma. Such a mutation might explain defects in bone, cartilage, or adipose tissue. Mutation in these cells, which arise from the common progenitor, might also disturb BM homeostasis.
Shortly before Garcia-Montero et al published their observations, we reported that clonal populations of MSCs from mastocytosis patients have impaired skeletal stem cell and hematopoietic support properties.4 We argued that dysfunctional mast cell/MSC interactions may drive epigenetic changes and pathological behavior in the MSC population. Based on Garcia-Montero's findings, this may be true in the majority of patients, but the authors have taken the story one step further. When they analyzed a significantly larger number of fresh BM samples from patients with SM than we did, they detected c-kit mutations in highly purified, early-passage MSCs in 1 of 4 patients. Although quantitative polymerase chain reaction (PCR) experiments were performed with appropriate controls, it is remotely possible that these cultures were still contaminated with small numbers of mast cells. In future experiments, one could eliminate this possibility by analyzing clones of cells derived from single MSCs or with single-cell PCR, but for now, it is interesting and instructive to take the authors’ conclusions at face value. They raise several important questions regarding the pathophysiology of mastocytosis as well as the possible connection between the developmental origin of skeletal stem cells and hematopoietic stem cells.
There are now a number of examples of BMSC-driven or dependent pathologies in the BM niche in myelodysplastic syndromes.5 Recent reports suggest that skeletal stem cells and hematopoietic cells in the BM cavity may have reciprocal interactions.6 BMSCs create a 3-dimensional network, and by secreting various growth factors and other signaling molecules, they play a central role in controlling the BM microenvironment in health and disease. How a c-kit mutation affects this regulatory function remains to be determined, and the difference between mutant BMSCs and wild-type BMSCs in mastocytosis patients should be explored.
In which kind of cell does the D816V mutation arise? The following possibilities should be considered (see figure). First, HSCs and BMSCs may be derived from a common stem/progenitor cell. If a mutation were to take place in this hypothetical stem cell, both hematopoietic and the mesenchymal cells would be expected to harbor the genetic defect. Although the existence of such “super” stem cells in the adult hematopoietic niche continues to be debated, there is a plausible candidate—the very small embryonic-like stem cells. These cells appear to have a differentiation potential that goes beyond the well-established lineage restriction of HSCs and BMSCs.7
Another possibility is that the mutation arises in a hematopoietic cell progenitor that can give rise to stromal cells. This may be a process that is analogous to the creation of myeloid-derived fibrocytes,8 many of which eventually stop expressing CD45, obscuring their hematopoietic cell origin. Although reports suggesting that cells might arise in this way are few in number, there is evidence that this sort of transformation could occur.9
Those scientists who are averse to the idea that mast cells and BMSCs might have a common precursor are left with a somewhat unattractive alternative—that c-kit mutations in the 2 cell populations occurred independently. If the putative mutation takes place in a BMSC progenitor, most sorts of BMSCs would be expected to have it. If it occurred in a more differentiated cell, the resulting clone might have a more narrow set of functions and resemble skeletal stem cells, pericytes, CAR cells,10 stromal fibroblasts, etc. It would certainly be interesting and important to study clonally selected mutant MSCs in more detail.
The existence of such cells would raise another possibility—that BMSCs could have gain-of-function mutations in diseases that share some similarities with mastocytosis, but lack mast cell pathology. Again, analysis of single cell–derived BMSC colonies or single-cell PCR studies of fresh uncultured BM-derived MSCs could readily address the questions listed here. The methods available to us have become so powerful that we should no longer be constrained by dogma. Rather than debating hypotheses like the ones we have outlined in this commentary, we should simply test them. It would be exciting to rewrite our textbooks.
Conflict-of-interest disclosure: The authors declare no competing financial interests.