In this issue of Blood, Brunstein et al report on “Umbilical cord blood–derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect.” This is an important study demonstrating the potential for adoptive regulatory T-cell (Treg) therapy as effective graft-versus-host disease (GVHD) prophylaxis.1
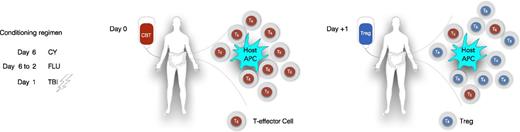
Distribution of infused CB Tregs into different compartments. APC, antigen-presenting cell; CBT, CB transplant; CY, cyclophosphamide (50 mg/kg); FLU, fludarabine (40 mg/m2); TBI, total body irradiation (200 cGy).
Distribution of infused CB Tregs into different compartments. APC, antigen-presenting cell; CBT, CB transplant; CY, cyclophosphamide (50 mg/kg); FLU, fludarabine (40 mg/m2); TBI, total body irradiation (200 cGy).
Brunstein et al have pioneered the use of cord blood (CB)-derived Tregs for the prevention of GVHD, with continued improvements in this transformative adoptive cell therapy. In their first clinical trial,2 they were not able to generate the planned Treg doses ex vivo for almost 20% of the patients and the acute GVHD rates were marginally better than in their historical controls. With improvements in the ex vivo expansion procedure, including the use of K562 antigen presenting expressing CD64 and CD86 in place of immunomagnetic beads expressing CD3 and CD28, and the refinements in the bead concentration described below, the investigators were able to generate CB Treg doses as high as 100 × 106/kg.1 In a double CB transplant setting with 4 to 6 antigen matches, the Minnesota group now reports important reductions in the acute GVHD rates (9% vs 45% in the controls (P = .05).
This study underscores, however, that good manufacturing practice–compliant cell therapy procedures can be difficult and require meticulous diligence to generate the desired product. In the current study, the investigators performed their validation runs using an open selection system with a conjugated anti-CD25 magnetic microbead concentration of 1:350. During the actual clinical trial, they used a closed system with the same bead concentration that lead to poor yields of CD25+ cells. Out of 13 Treg products that were manufactured, 11 were infused. Five products did not reach the target dose and 2 products failed to achieve the minimum required dose of 3 × 106/kg. The investigators changed the bead concentration mid-study, which then allowed them to expand the CB Tregs successfully.
Another important observation in this study was that the high doses of infused Tregs did not result in longer persistence in the peripheral blood (PB) of the patients. In fact, no Tregs were detected beyond day 14 post-infusion in any of the patients. The absence of CB Tregs in the PB allows for the possibility that they may have migrated to the tissues (see figure). The infused CB Tregs could home to the tissues that were minimally or more extensively inflamed from the preparative regimen, and engage with the host antigen-presenting cell to abrogate their interaction with the T-effector cells and thus prevent the “initiation phase” of GVHD.
Several approaches are under investigation to enhance Treg efficacy by increasing their potency with pharmacologic inhibition of protein kinase C-θ,3 augmentation of signal transducer and activator of transcription (Stat)5 signaling,4 and inhibition of Stat1.5 Because all-trans retinoic acid has been shown to significantly augment the suppressive effects on xenogenic GVHD6 and rapamycin has been shown to stabilize infused Treg,7 the addition of these agents to Treg cultures are being explored. Manipulating the trafficking of CB Tregs using fucosylation to improve their homing toward sites of inflammation is also under investigation.8
Although the patient numbers are very small and this study needs to be confirmed with larger cohorts, the impressive decrease in the incidence of grade 2/4 acute GVHD as a result of adoptive therapy with CB Tregs has created a new benchmark in this setting. It is important to note that no increase in relapse, infection, or toxicity was observed even at highest Treg doses of 100 × 106/kg. Di Ianni et al have also shown safety of PB-derived Tregs for the prevention of GVHD in the haplo-identical donor transplant setting9 and low risk of relapse on long-term follow up.10
In summary, the successful translation of CB Treg adoptive therapy to the clinic is a major step forward for stem cell transplantation, by reducing the risk of GVHD. With this approach, Brunstein et al have paved the way for of the application of Treg therapy in other risk transplant settings, including haplo-identical and one antigen-mismatched unrelated donor transplants.
Conflict-of-interest disclosure: The authors declare no competing financial interests.