Key Points
OCs play a crucial role in myeloma-induced immunosuppressive microenvironment.
Therapeutic anti-CD38 mAb partially overcomes the immunosuppressive effect of OCs.
Abstract
The number and activity of osteoclasts (OCs) are strongly enhanced by myeloma cells, leading to significant bone lesions in patients with multiple myeloma (MM). Mechanisms remain elusive as to whether myeloma-supporting OCs also induce suppressive immune bone marrow (BM) microenvironment. Here, we first show that OCs significantly protect MM cells against T-cell–mediated cytotoxicity via direct inhibition of proliferating CD4+ and CD8+ T cells. The immune checkpoint molecules programmed death ligand 1 (PD-L1), Galectin-9, herpesvirus entry mediator (HVEM), and CD200, as well as T-cell metabolism regulators indoleamine 2, 3-dioxygenase (IDO), and CD38 are significantly upregulated during osteoclastogenesis. Importantly, the levels of these molecules, except CD38, are higher in OCs than in MM cells. Anti–PD-L1 monoclonal antibody (mAb) and IDO inhibitor partly overcome OC-inhibited T-cell responses against MM cells, confirming their roles in OC-suppressed MM cell lysis by cytotoxic T cells. In addition, Galectin-9 and a proliferation-induced ligand (APRIL), secreted by OCs, are significantly upregulated during osteoclastogenesis. Galectin-9 specifically induces apoptosis of T cells while sparing monocytes and MM cells. APRIL induces PD-L1 expression in MM cells, providing additional immune inhibition by OCs. Moreover, CD38 is significantly upregulated during osteoclastogenesis. When targeted by an anti-CD38 mAb, suppressive T-cell function by OCs is alleviated, associated with downregulation of HVEM and IDO. Taken together, these results define the expression of multiple immune proteins and cytokines in OCs essential for suppressive MM BM milieu. These results further support the combination of targeting these molecules to improve anti-MM immunity.
Introduction
Osteolytic bone disease affects 80% of multiple myeloma (MM) patients, with negative impact on both quality of life and overall survival.1 A bidirectional prosurvival regulatory loop exists between osteoclasts (OCs) and MM cells in the bone marrow (BM) microenvironment.2 In addition to their major function in bone remodeling, OCs have been recently implicated in multiple complex functions.3,4 They can regulate the immune system (and this relationship is usually termed as “osteoimmunology”). Specifically, osteoclastic bone resorption is associated with T-cell immune activation in autoimmune disease through crosstalk between OCs and T cells.5 The activity of OCs must be tightly controlled in order to balance between bone deposition and degradation. Activated T cells induce osteoclastogenesis via production of potent osteoclastogenic cytokines, receptor activator of nuclear factor-κB ligand (RANKL) and interleukin-1b (IL-1b).6 In parallel, activated T cells inhibit OC differentiation via secretion of interferon-γ (IFN-γ), IL-4, and IL-10.5 Although the reciprocal impact of OCs on T cells is less defined, OCs effectively suppress T-cell proliferation in a feedback loop mechanism to prevent osteoporosis or osteosclerosis.7 In fact, the suppression of T cells occurs from the beginning of OC formation. For example, CD200 expression is significantly upregulated prior to fusion of proliferating monocytes and subsequently enhances RANKL signaling, which promotes fusion.8 Meanwhile, an inhibitory CD200 receptor (CD200R) is induced by lymphoid cells, ie, natural killer and activated T cells.9 The dual function of CD200 suggests the existence of an “OC checkpoint,” which downregulates immune effector cells. Here, we postulated that this OC checkpoint mechanism may promote immune escape of MM cells, analogous to tumor cells evading immune destruction due to aberrant immune checkpoint pathways.
Various monocyte-derived cells, including macrophages, myeloid-derived suppressor cells (MDSCs), and dendritic cells (DCs), have been implicated in T-cell suppression in MM.10-12 They are recruited by MM cells to create a localized immunosuppressive niche for MM survival. OCs are terminally differentiated cells of the monocyte/macrophage lineage with similar immune receptors in the innate immune system.4 Recently, OCs were reported to act as antigen-presenting cells (APCs) to activate T cells.13 In MM, APCs (macrophages and plasmacytoid DCs) are increased and contribute to immune dysfunction in the BM microenvironment.12,14 We thus hypothesized that the OC–T-cell crosstalk, analogous to the interaction between APCs and T cells, may regulate immune-bone interactions in MM. Furthermore, bones are a common site of treatment-resistant infections and metastatic cancers, highlighting an impaired immune response in the bone microenvironment. Because defective T-cell function is a key mechanism of tumor evasion from immunologic surveillance,15 we investigated here the immunosuppressive function of OCs in adaptive immunity in MM.
Material and methods
Patient samples and cell lines
All CD138+ MM cell lines were cultured as described previously.16 Patient MM samples were obtained after informed consent, in accordance with the Declaration of Helsinki and under the auspices of a Dana-Farber Cancer Institute (DFCI) Institutional Review Board-approved protocol. CD138+ plasma cells from MM patients were purified by CD138 MicroBeads (Miltenyi Biotech, Auburn, CA).
Generation of OCs
Peripheral blood mononuclear cells (PBMCs) were obtained from MM patients and normal donor samples by Ficoll-Paque (GE Healthcare) density gradient centrifugation. PBMCs from MM patients or CD14+ monocytes from healthy donors’ PBMCs were plated in 12-well plates (Corning, NY) at 2 × 106/mL per well in OC medium, composed of RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (HyClone; GE Healthcare Life Sciences), penicillin-streptomycin (Gibco, Life Technologies), human recombinant RANKL (50 ng/mL; Miltenyi Biotec), and human recombinant macrophage colony-stimulating factor (M-CSF) (25 ng/mL; Miltenyi Biotec).17 Medium with cytokines was changed every 3rd day. After 14 days in culture, multinucleated OCs were generated and used for subsequent experiments. Tartrate-resistant acid phosphatase (TRAP) staining of OCs was performed with the Leukocytes Acid Phosphatase Kit (Sigma-Aldrich).
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
RNA was extracted from OCs and MM cells, and messenger RNA (mRNA) expression was quantified using the ViiA7 Real-Time PCR System and analyzed using version 1.2 software (Life Technologies).
Immunophenotyping
Immunofluorescence analysis was performed using a BD FACSCanto II flow cell analyzer with FACSDiva version 5.0 acquisition/analysis software (BD Biosciences). Data were analyzed using FloJo version 8.6.6 (Tree Star Inc). Anti-CD14 (APC, M5E2), antiprogrammed death ligand 1 (PD-L1) (Brilliant Violet 421, 29E.2A3), and anti-CD38 (Brilliant Violet 421, HIT2) antibodies (Ab) were obtained from BioLegend (San Diego, CA). Antiherpesvirus entry mediator (HVEM) (Brilliant Violet 421, CW10) and anti-CD200 (Brilliant Violet 421, MRC OX-104) were obtained from BD Biosciences.
Immunoblotting
Anti–PD-L1, anti-indoleamine 2, 3-dioxygenase (IDO), antiphosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2), and antiphosphorylated mitogen-activated protein kinase 1/2 (p-MEK1/2) Abs were obtained from Cell Signaling Technology (Danvers, MA); anti-HVEM Ab and anti-CD38 Ab from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-CD200, anti–Galectin-9, and anti-inducible T-cell co-stimulator Ligand Ab from Abcam (Cambridge, MA).
Transfection
MM cells were transfected with PD-L1, B-cell maturation antigen (BCMA), or APRIL-expression plasmids (ccsbBroad304_03086; PLOHS_ccsbBEn_05884; PLOHS_100067106), or control vector plasmid using the Thermo Scientific Trans-Lentiviral Packaging System. Two days after transfection, MM cells overexpressing PD-L1, BCMA, APRIL, or control vector (RPMI8226-pLoC–PD-L1; RPMI8226-pLoC-BCMA; RPMI8226-pLoC-APRIL) were treated with blasticidin (5 μg/mL; Invitrogen) to select for stable transfectants, which were validated with flow cytometry.
Generation of MM-specific cytotoxic T lymphocytes (CTLs)
For generating CTLs, 5 × 106 nonadherent PBMCs from normal individuals were cocultured with 5 × 105 irradiated (20 000 rad) KMS-28BM cells for 7 days in medium containing RPMI 1640 supplemented with L-glutamine (2 mm), penicillin (100 mg/mL), streptomycin (100 mg/mL), 15% human AB serum, and IL-2 (20 U/mL; R&D Systems). After Ficoll-Hypaque centrifugation, viable cells (5 × 105/mL) were cultured and re-stimulated with irradiated KMS-28 BM cells. After 7 days, CD8+ T cells were purified using magnetic-activated cell sorting. CTLs obtained were subsequently cocultured with target cells KMS-28BM; anti–PD-L1 antibody (R&D Systems) and 1-methyl-dl-tryptophan (Trp) (Sigma-Aldrich) were added to block the PD-L1 and IDO-mediated signal, respectively. After 4 hours, cytotoxicity was evaluated by measuring lactate dehydrogenase (LDH) activity using CytoTox96 nonradioactive assay (Promega, Madison, WI).
Immunofluorescence analysis
Monocytes or OCs were fixed with 1% paraformaldehyde and later stained with indicated Abs to detect PD-L1, IDO, Galectin-9, HVEM, and CD200 expression. The nuclei were detected with 4′,6-diamidino-2-phenylindole staining.
Enzyme-linked immunosorbent assay (ELISA)
Precoated plates of human Galectin-9 and IDO-specific sandwich ELISA (R&D Systems, Minneapolis, MN; LifeSpan BioSciences, Seattle, WA) were used to detect Galectin-9 and IDO levels.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Differences in means were tested for significance using a 2-sided Student t test to evaluate continuous variables of 2 groups, and with one-way ANOVA to evaluate continuous variables of more than 2 groups. P < .05 represented statistical significance.
Results
OCs rescue CTL-induced apoptosis of myeloma cell line cells
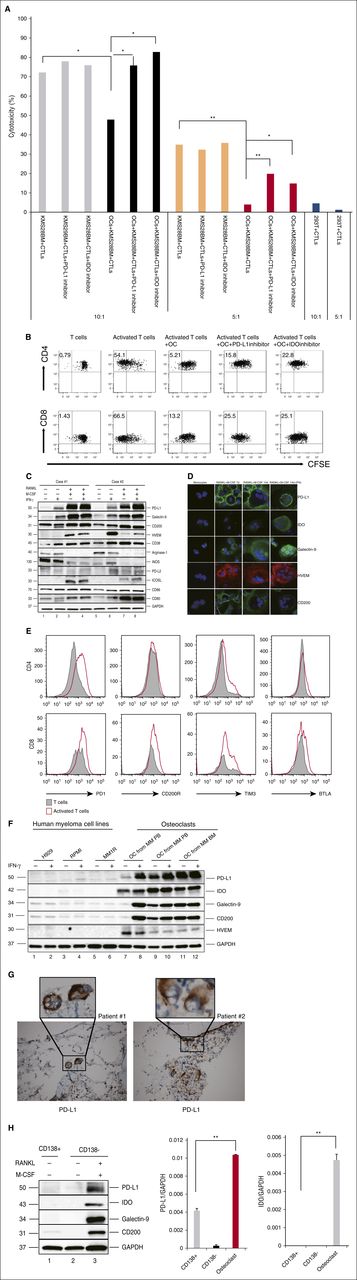
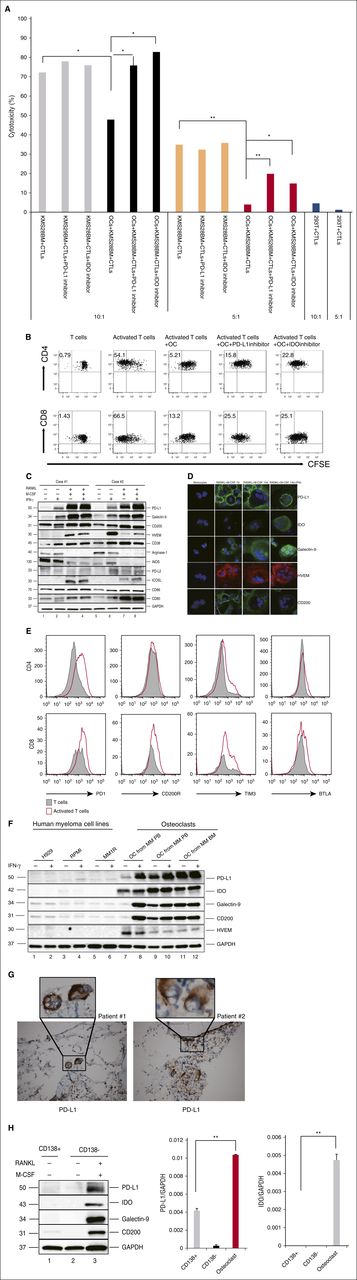
First, we examined if OCs could modulate T-cell–mediated anti-MM immunity. OCs and KMS28-BM cell-specific CTLs were generated from the same healthy donor. KMS28-BM cells were then cultured with KMS28-BM cell-specific CTLs in 96-well plates for 4 hours, with or without OCs. The specificity of CTLs was confirmed by coculturing KMS28-BM cell-specific CTLs and 293T cells (kidney tumor). When the CTL/KMS28-BM ratio was 10, 72.1% KMS28-BM cells were killed by CTLs without OCs, whereas 47.8% of KMS28-BM cells were killed in the presence of OCs. When the CTL/KMS28-BM ratio was 5, 34.9% KMS28-BM cells were killed by CTLs without OCs, whereas 3.9% KMS28-BM cells were killed in the presence of OCs. Importantly, this protection was partly abrogated by PD-L1 Ab and IDO inhibition (Figure 1A).
OCs protect MM cells against T-cell–mediated cytotoxicity by upregulating expression of multiple co-inhibitory molecules. (A) OCs and MM-specific CTLs were generated from the same healthy donor. CTLs were cocultured with target cells (KMS28-BM) in the absence or presence of OCs with or without the PD-L1 inhibitor (10 μg/mL)/IDO inhibitors (1-methyl-dl-Trp, 1 mM). After 4 hours, cytotoxicity was evaluated by measuring LDH activity in the supernatants. Shown is mean ± SD of the 3 representative independent experiments. (B) Proliferation of T cells stimulated by anti-CD2/CD3/CD28 beads (T:Bead ratio of 1:1) in the absence or presence of autologous OCs for 5 days was measured with CFSE dilution assay. (C-D) CD14+ monocytes were cocultured with RANKL and M-CSF for 14 days, and OCs were identified by TRAP staining. OCs were also cocultured with IFN-γ (20 IU/mL) for 12 hours. Protein expression in monocytes and OCs were determined by immunoblotting (C) and immunofluorescence (D). (E) T cells stimulated by anti-CD2/CD3/CD28 beads (T:Bead ratio of 1:1). Expression of PD-1, CD200R, Tim-3, and BTLA were examined by flow cytometry. (F) OCs were cultured from PBMCs or BM mononuclear cells from MM patients without CD14 selection. Levels of inhibitory molecules were significantly higher in OCs than in MM cells. (G) IHC analysis of 2 representative BM specimens from MM patients shows PD-L1 expression (brown) on OCs. Original magnification: ×20 (×100 in insets). (H) The expression of PD-L1, IDO, Galectin-9, and CD200 in CD138+ cells, CD138− cells, and OCs from the same patient was determined by immunoblotting and real-time qRT-PCR. *P < .05; **P < .001; by unpaired 2-sided Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICOSL, inducible T-cell co-stimulator ligand; IHC, immunohistochemistry; PB, peripheral blood.
OCs protect MM cells against T-cell–mediated cytotoxicity by upregulating expression of multiple co-inhibitory molecules. (A) OCs and MM-specific CTLs were generated from the same healthy donor. CTLs were cocultured with target cells (KMS28-BM) in the absence or presence of OCs with or without the PD-L1 inhibitor (10 μg/mL)/IDO inhibitors (1-methyl-dl-Trp, 1 mM). After 4 hours, cytotoxicity was evaluated by measuring LDH activity in the supernatants. Shown is mean ± SD of the 3 representative independent experiments. (B) Proliferation of T cells stimulated by anti-CD2/CD3/CD28 beads (T:Bead ratio of 1:1) in the absence or presence of autologous OCs for 5 days was measured with CFSE dilution assay. (C-D) CD14+ monocytes were cocultured with RANKL and M-CSF for 14 days, and OCs were identified by TRAP staining. OCs were also cocultured with IFN-γ (20 IU/mL) for 12 hours. Protein expression in monocytes and OCs were determined by immunoblotting (C) and immunofluorescence (D). (E) T cells stimulated by anti-CD2/CD3/CD28 beads (T:Bead ratio of 1:1). Expression of PD-1, CD200R, Tim-3, and BTLA were examined by flow cytometry. (F) OCs were cultured from PBMCs or BM mononuclear cells from MM patients without CD14 selection. Levels of inhibitory molecules were significantly higher in OCs than in MM cells. (G) IHC analysis of 2 representative BM specimens from MM patients shows PD-L1 expression (brown) on OCs. Original magnification: ×20 (×100 in insets). (H) The expression of PD-L1, IDO, Galectin-9, and CD200 in CD138+ cells, CD138− cells, and OCs from the same patient was determined by immunoblotting and real-time qRT-PCR. *P < .05; **P < .001; by unpaired 2-sided Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ICOSL, inducible T-cell co-stimulator ligand; IHC, immunohistochemistry; PB, peripheral blood.
To further confirm that OCs can protect against T-cell responses, we cultured OCs with T cells from the same healthy donor in vitro for 4 days and assessed T-cell proliferation using carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay. Both CD4 and CD8 T-cell proliferation induced by anti-CD2/CD3/CD28 beads was significantly inhibited in the presence of OCs (Figure 1B). Such OC-inhibited T-cell proliferation was partly overcome by an inhibitory PD-L1 Ab and IDO inhibitor.
Inhibitory immune molecules are upregulated during osteoclastogenesis
To identify molecules mediating the inhibition of OCs on T-cell proliferation, we next examined the expression of immune-related proteins during osteoclastogenesis by immunoblotting. Because OCs can function as APCs,13 we measured CD80/86 molecules, which are expressed by APCs and involved in T-cell co-stimulation. CD80 expression was significantly induced, whereas CD86 was slightly upregulated in OCs compared with monocytes (Figure 1C). Because tumor cells use immune-checkpoint pathways as a major mechanism of immune resistance and APCs express similar molecules, we analyzed the expression of common checkpoint regulators. PD-L1, HVEM, Galectin-9, and CD200 proteins were significantly induced in OCs, as determined by immunoblotting (Figure 1C) and immunofluorescence (Figure 1D). PD-L1, Galectin-9, and HVEM expression was further enhanced by an additional 12-hour incubation of IFN-γ (20 IU/mL), a major inducer of co-inhibitory molecules secreted by activated T cells. It was also shown that activated T cells expressed a high level of programmed death 1 (PD-1), CD200R, Tim-3, and B and T lymphocyte attenuator (BTLA) (Figure 1E), which further confirmed the role of PD-1/PD-L1, CD200/CD200R, Galectin-9/Tim-3, and HVEM/BTLA pathway in OC-induced immunosuppression.
Previous studies have suggested that immune-checkpoint molecules are overexpressed in both MM cells and accessory cells in their microenvironment. We next compared the expression of PD-L1, Galectin-9, and HVEM on human MM cells and OCs. All these molecules were expressed at higher levels in OCs than in MM cells (Figure 1F). Immunohistochemistry analysis further confirmed PD-L1 expression in OCs from BM specimens from 2 representative MM patients (Figure 1G). Expression of PD-L1, Galectin-9, and CD200, as well as IDO, was further compared in CD138+ cells, CD138− cells, and OCs from the same patient. Their protein and mRNA expression was significantly higher in OCs than in patient MM cells (Figure 1H).
IDO is induced during OC formation
Immune inhibitory molecules also include certain metabolic enzymes, such as Arginase-1, inducible nitric oxide synthase (iNOS), and IDO. Although Arginase-1 expression and upregulation of iNOS are involved in MDSC-mediated T-cell suppression,18 neither Arginase-1 nor iNOS was upregulated in OCs (Figure 1C). This data suggested a preferential regulation of these molecules in immune-cell responses by MDSCs vs OCs. Expression of IDO in OCs was further enhanced following IFN-γ stimulation for 12 hours (Figure 1D). OCs cultured from MM patient PBMCs or BM mononuclear cells expressed a high level of IDO without IFN-γ stimulation in the presence of T lymphocytes (Figure 1F). OCs also expressed higher IDO than CD138+ MM cells from the same patient sample, as confirmed by immunoblotting and real-time qRT-PCR (Figure 1H). IDO upregulation in OCs was confirmed by real-time qRT-PCR (see supplemental Figure 1A, available on the Blood Web site). IDO levels were significantly increased in MM patient BM plasma (supplemental Figure 1B).
OC precursors (OCPs) also express inhibitory molecules
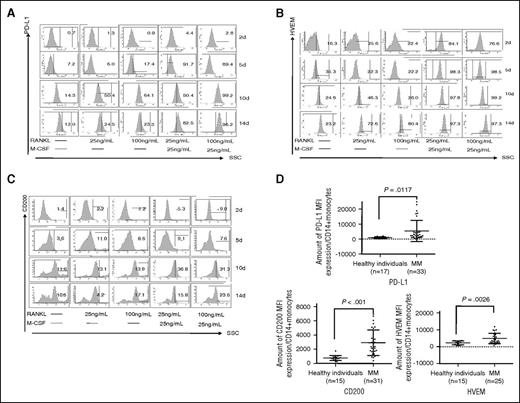
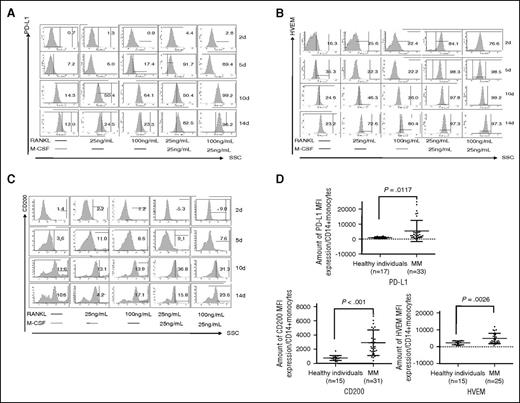
We also noticed that precursor cells (ie, OCPs), which are CD14+ monocytes stimulated with M-CSF and RANKL for 7 days without multinuclei, showed increased expression of PD-L1, Galectin-9, HVEM, and CD200 proteins (Figure 1D). Time-dependent upregulation of PD-L1, HVEM, and CD200 were next confirmed by flow cytometry (Figure 2A-C). M-CSF/RANKL-activated monocytes began to express PD-L1, HVEM, and CD200 after 5 days, with peak expression following 14 days. RANKL alone stimulated the expression of PD-L1, HVEM, and CD200, which was enhanced by M-CSF. Thus, OCPs, or immature OCs, express co-inhibitory molecules even before fusion to form >3 multinucleated OCs.
Time-course and dose-dependent analysis of inhibitory molecule expression during OC differentiation. (A-C) CD14+ monocytes were stimulated by RANKL and/or M-CSF followed by flow cytometry for PD-L1, HVEM, and CD200 at day 2, day 5, day 10, and day 14. (D) Monocytes were collected from healthy donors and MM patients to examine PD-L1, HVEM, and CD200 by flow cytometry. *P < .05; **P < .001; by unpaired 2-sided Student t test. SSC, side scatter.
Time-course and dose-dependent analysis of inhibitory molecule expression during OC differentiation. (A-C) CD14+ monocytes were stimulated by RANKL and/or M-CSF followed by flow cytometry for PD-L1, HVEM, and CD200 at day 2, day 5, day 10, and day 14. (D) Monocytes were collected from healthy donors and MM patients to examine PD-L1, HVEM, and CD200 by flow cytometry. *P < .05; **P < .001; by unpaired 2-sided Student t test. SSC, side scatter.
Monocytes and monocyte-derived cells contribute to the suppression of host antitumor immunity in solid tumors.19 Moreover, monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1.20 We also found that PDL1, HVEM and CD200 expression on monocytes of newly diagnosed MM patients was significantly increased compared with those from healthy individuals (5453 ± 1215 vs 988 ± 127 mean fluorescence intensity [MFI] for PD-L1, P = .0117; 4881 ± 597 vs 2238 ± 298 MFI for HVEM, P = .0026; and 2917 ± 320 vs 769 ± 89 MFI for CD200, P < .001) (Figure 2D).
Galectin-9 induces apoptosis of human T cells but not MM cell line cells
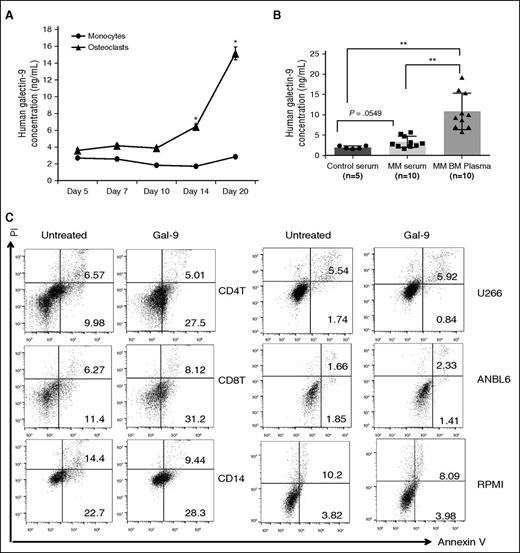
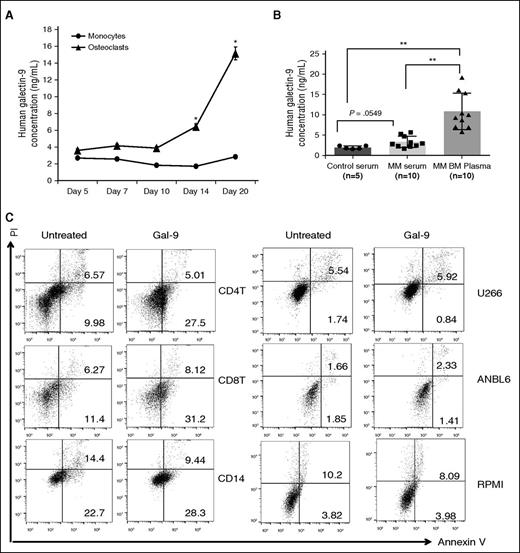
Because of their ability to induce local immunosuppression, members of the Galectin family are emerging as a new mechanism of T-cell exhaustion.21 Galectin-9 is expressed in the cytoplasm, on the plasma membrane, and is excreted.22 We observed intracellular and cell surface Galectin-9 expression by immunoblotting (Figure 1C,F,H) and immunofluorescence (Figure 1D), respectively. Galectin-9 secretion was significantly increased in the supernatant of OCs vs monocytes (Figure 3A). Mean levels of Galectin-9 in MM patient serum (n = 10) (3.342 ± 0.442 ng/mL) were higher than in healthy donor serum (1.977 ± 0.175 ng/mL) (Figure 3B). It was significantly higher in BM plasma of MM patients (10.880 ± 1.413 ng/mL) than in serum from healthy donors (P = .04) (Figure 3B).
Galectin-9 preferentially induces apoptosis of lymphoid T cells but not myeloid cells and MM cell line cells. (A) CD14+ monocytes were stimulated with RANKL and M-CSF. Supernatant was collected to measured Galectin-9 by ELISA, (B) serum was obtained from 5 healthy donors, and simultaneous serum and BM plasma were obtained from 10 MM patients. Galectin-9 level was determined by ELISA. (C) PBMCs from healthy donors and 3 MM cells were treated with recombinant Galectin-9 (1 μg/mL) for 12 hours, stained with Annexin V/PI, and analyzed by flow cytometry. Bottom right quadrants represent early apoptotic cells (Annexin V-positive only) and top right quadrant the late apoptotic cells (Annexin V- and PI-positive). *P < .05; **P < .001; by unpaired 2-sided Student t test. PI, propidium iodide.
Galectin-9 preferentially induces apoptosis of lymphoid T cells but not myeloid cells and MM cell line cells. (A) CD14+ monocytes were stimulated with RANKL and M-CSF. Supernatant was collected to measured Galectin-9 by ELISA, (B) serum was obtained from 5 healthy donors, and simultaneous serum and BM plasma were obtained from 10 MM patients. Galectin-9 level was determined by ELISA. (C) PBMCs from healthy donors and 3 MM cells were treated with recombinant Galectin-9 (1 μg/mL) for 12 hours, stained with Annexin V/PI, and analyzed by flow cytometry. Bottom right quadrants represent early apoptotic cells (Annexin V-positive only) and top right quadrant the late apoptotic cells (Annexin V- and PI-positive). *P < .05; **P < .001; by unpaired 2-sided Student t test. PI, propidium iodide.
Galectin-9 induces T-cell apoptosis and a study suggested that Galectin-9 has anti-MM activity.23 We observed here that incubation of CD4+ and CD8+ T cells with Galectin-9 (1 μg/mL) for 12 hours in 10% FBS RPMI 1640 medium with IL-2 (24 IU/mL) significantly increased apoptosis (9.98 + 0.14% to 27.5 + 1.34%; 11.4 + 0.78% to 31.2 + 2.04%, respectively). In contrast, Galectin-9 had no impact on the fraction of apoptotic cells in CD14+ cells and MM cell line cells (Figure 3C). These results confirmed the specific proapoptotic effect of Galectin-9 against lymphoid T cells.
A proliferation-inducing ligand (APRIL), highly expressed in OCs, induces PD-L1 expression in MM cell line cells
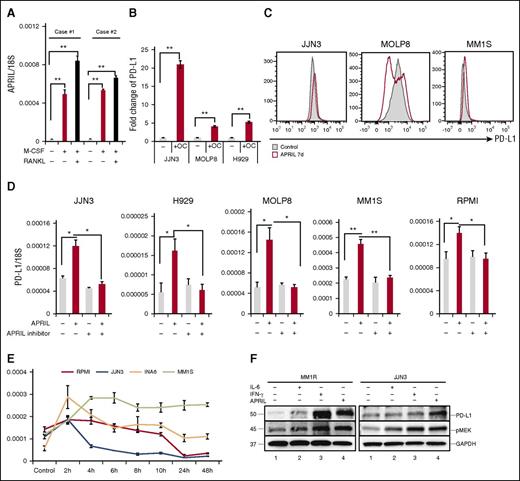
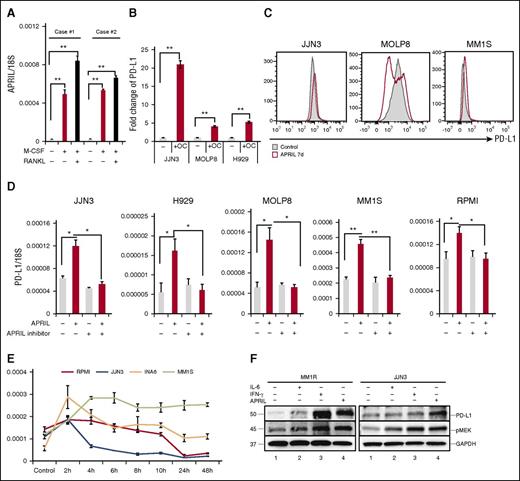
Several studies have indicated that myeloid cells and OCs are the main source of APRIL production in the BM environment.24,25 Specifically, we find APRIL stimulates human MM growth, chemoresistance, and immunosuppression in the BM microenvironment.26 In accordance with previous reports, APRIL was upregulated in OCs than monocytes (Figure 4A). We next performed Transwell experiments to determine whether OCs modulate PD-L1 expression on MM cell line cells via an APRIL-dependent manner. PD-L1 expression was increased when MM cell lines cells were cocultured with OCs (Figure 4B). APRIL-induced PD-L1 expression was further confirmed using flow cytometry (Figure 4C). Importantly, APRIL stimulation enhanced PD-L1 expression in all tested MM cell lines; conversely, anti-APRIL mAbs blocked PD-L1 induction (Figure 4D). Time-course analysis of PD-L1 mRNA levels following APRIL stimulation showed that PD-L1 reached maximal levels in 2 to 6 hours (Figure 4E). IFN-γ and IL-6 have also been reported to induce PD-L1 expression in MM cells.27,28 We next compared the effect of IFN-γ, IL-6, and APRIL on PD-L1 expression by immunoblotting. All these cytokines enhanced PD-L1 expression in MM1R and JJN3 MM cells, associated with cytokine-induced phosphorylation of MEK1/2 (Figure 4F).
APRIL induces PD-L1 expression on human MM cell line cells mainly via paracrine mechanism. (A) CD14+ monocytes were stimulated with M-CSF and/or RANKL for 14 days. APRIL expression in these cells was examined by real-time qRT-PCR; 18S was used to normalize APRIL expression. (B) Transwell experiments were performed in which MM cells were placed in the upper chamber and medium alone, or OCs were placed in the lower chambers. After 4 days, mRNA was collected from MM cells and subjected to real-time qRT-PCR for APRIL normalized to 18S. Fold increases compared with controls were shown. (C) MM cell lines were cultured with APRIL (200 ng/mL) for 7 days and PD-L1 expression was examined by flow cytometry. (D) MM cell lines were treated with human recombinant APRIL and/or anti-APRIL mAb (200 ng/mL) for 4 hours, and PD-L1 expression was examined by real-time qRT-PCR. (E) Indicated MM cell lines were stimulated with APRIL and PD-L1 expression was examined by real-time qRT-PCR. (F) MM1R and JJN3 cells were cultured with IFN-γ (500 IU/mL, 24 hours), IL-6 (10 ng/mL, 48 hours), and APRIL (200 ng/mL, 7 days). PD-L1 and pMEK1/2 expression was assessed by immunoblotting of cell lysates. *P < .05; **P < .001; by unpaired 2-sided Student t test.
APRIL induces PD-L1 expression on human MM cell line cells mainly via paracrine mechanism. (A) CD14+ monocytes were stimulated with M-CSF and/or RANKL for 14 days. APRIL expression in these cells was examined by real-time qRT-PCR; 18S was used to normalize APRIL expression. (B) Transwell experiments were performed in which MM cells were placed in the upper chamber and medium alone, or OCs were placed in the lower chambers. After 4 days, mRNA was collected from MM cells and subjected to real-time qRT-PCR for APRIL normalized to 18S. Fold increases compared with controls were shown. (C) MM cell lines were cultured with APRIL (200 ng/mL) for 7 days and PD-L1 expression was examined by flow cytometry. (D) MM cell lines were treated with human recombinant APRIL and/or anti-APRIL mAb (200 ng/mL) for 4 hours, and PD-L1 expression was examined by real-time qRT-PCR. (E) Indicated MM cell lines were stimulated with APRIL and PD-L1 expression was examined by real-time qRT-PCR. (F) MM1R and JJN3 cells were cultured with IFN-γ (500 IU/mL, 24 hours), IL-6 (10 ng/mL, 48 hours), and APRIL (200 ng/mL, 7 days). PD-L1 and pMEK1/2 expression was assessed by immunoblotting of cell lysates. *P < .05; **P < .001; by unpaired 2-sided Student t test.
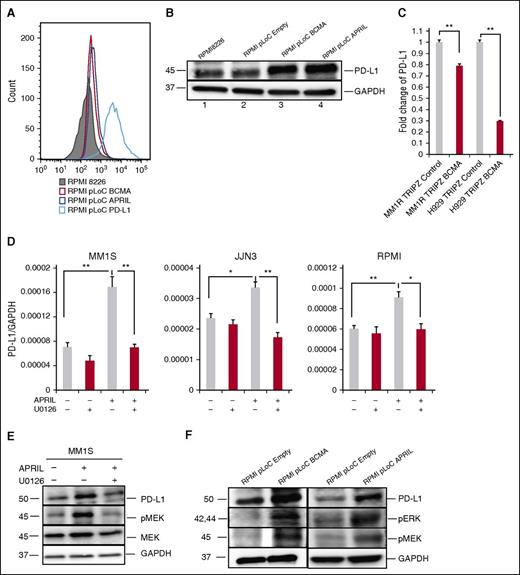
APRIL/BCMA pathway regulates PD-L1 expression in MM cell line cells
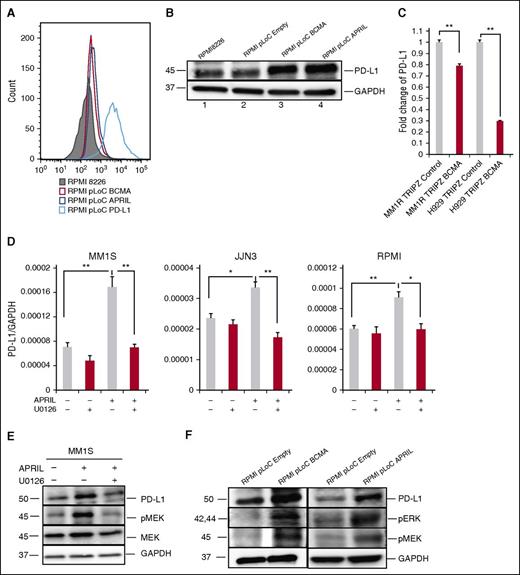
Because BCMA is a receptor for APRIL, we determined the role of the APRIL/BCMA pathway in regulating the expression of PD-L1 by overexpressing BCMA or APRIL in RPMI 8226 cells. BCMA overexpression significantly enhanced PD-L1 expression as validated by flow cytometry and immunoblotting (Figure 5A-B), confirming our previous findings.26 Overexpression of APRIL in RPMI 8226 cells using APRIL-specific plasmid (RMPI8226-pLoC-APRIL) also induced PD-L1 protein expression. These results reconfirm that PD-L1 induction in MM cells is dependent on APRIL/BCMA signaling cascade. Conversely, knockdown of BCMA expression in MM1R and H929 MM cells using BCMA-short hairpin RNA lentiviral particles significantly downregulated PD-L1 expression (Figure 5C).
The MEK/ERK pathway plays an important role in APRIL-induced PD-L1 expression in MM cells. (A-B) PD-L1 expression was examined by flow cytometry (A) and immunoblotting (B) in indicated RPMI 8226 transfectants. (C) PD-L1 mRNA expression was examined in BCMA knockdown (MM1R-TRIPZ-BCMA and H929-TRIPZ-BCMA) vs control MM cells by real-time qRT-PCR. Fold changes of PD-L1/18S to control were shown. (D) MM cells were treated with APRIL and/or U0126 molecules (100 nM) for 4 hours. PD-L1 expression was examined using real-time qRT-PCR; (F) PD-L1, pERK, and pMEK was examined by immunoblotting in indicated cells. (E) Serum-starved MM1S cells were pretreated with U0126 (100 nM) followed by APRIL stimulation. Cell lysate was collected and subjected to immunoblotting with indicated Abs. Shown is mean ± SD from 3 independent experiments. *P < .05; **P < .001; by unpaired 2-sided Student t test.
The MEK/ERK pathway plays an important role in APRIL-induced PD-L1 expression in MM cells. (A-B) PD-L1 expression was examined by flow cytometry (A) and immunoblotting (B) in indicated RPMI 8226 transfectants. (C) PD-L1 mRNA expression was examined in BCMA knockdown (MM1R-TRIPZ-BCMA and H929-TRIPZ-BCMA) vs control MM cells by real-time qRT-PCR. Fold changes of PD-L1/18S to control were shown. (D) MM cells were treated with APRIL and/or U0126 molecules (100 nM) for 4 hours. PD-L1 expression was examined using real-time qRT-PCR; (F) PD-L1, pERK, and pMEK was examined by immunoblotting in indicated cells. (E) Serum-starved MM1S cells were pretreated with U0126 (100 nM) followed by APRIL stimulation. Cell lysate was collected and subjected to immunoblotting with indicated Abs. Shown is mean ± SD from 3 independent experiments. *P < .05; **P < .001; by unpaired 2-sided Student t test.
APRIL-induced PD-L1 expression via a MEK/ERK pathway
To further elucidate the signaling pathway mediating APRIL/BCMA-induced PD-L1 expression in MM cells, we next cultured MM cell line cells stimulated with APRIL in the presence of several transduction pathway inhibitors. A MEK1/2 inhibitor, U0126, abrogated APRIL-induced PD-L1 expression in all MM cell line cells (Figure 5D). Blocking of nuclear factor-κB and phosphatidylinositol 3-kinase pathways with SN50 and LY294002, respectively, showed minimal effect on APRIL-induced PD-L1 expression (data not shown). In addition, APRIL and BCMA overexpressing RPMI 8226 transfectants show higher expression of pERK1/2, pMEK1/2, and PD-L1 proteins (Figure 5E), which was also confirmed in MM1S cells (Figure 5F). These data indicate that the MEK/ERK pathway played an important role in APRIL-induced PD-L1 expression.
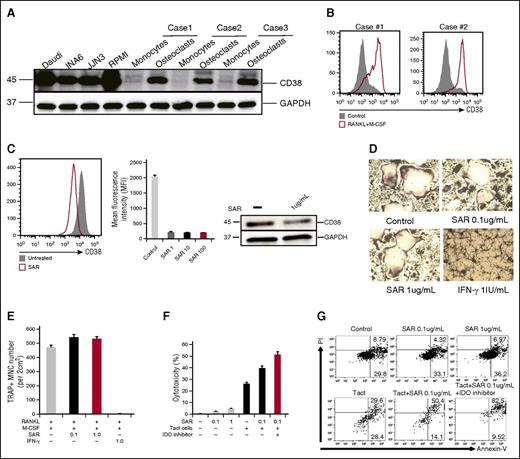
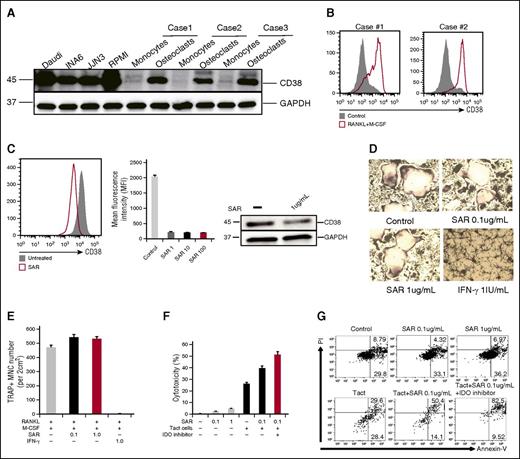
CD38 is induced during OC formation
Besides bone remodeling, OCs are also involved in the mobilization of calcium from bones. Because CD38 is an ectoenzyme (cyclic adenosine 5′-diphosphate ribosylhydrolase) regulating the intracytoplasmic concentration of calcium, we therefore analyzed CD38 on OCs by immunoblotting. CD38, which is frequently expressed in MM cells, is also induced during OC formation, as analyzed by immunoblotting (Figure 1C and 6A) and flow cytometry (Figure 6B). Because anti-CD38 mAbs recently showed a clinical benefit in relapsed and refractory MM patients,29,30 we further addressed whether an anti-CD38 mAb SAR650984 (SAR)31 has any impact on OCs. We cultured CD14+ monocytes with RANKL and M-CSF in 10% RPMI medium for 7 days ex vivo and SAR was then added. CD38 expression on OCs was analyzed at day 14. SAR significantly reduced CD38 expression on OCs (Figure 6C), whereas minimal adverse effects were seen using caspase 7, caspase 8, and CellTiter-Glo assays (supplemental Figure 2A). Using TRAP assay, SAR showed no impact on multinucleated OC formation, whereas IFN-γ strongly inhibited OC formation (Figure 6D-E).
The impact of anti-CD38 Ab on OC differentiation from CD14-purified monocytes ex vivo. (A) CD38 was examined by immunoblotting in indicated cells. (B) CD38 expression was examined by flow cytometry during osteoclastogenesis. (C) Purified CD14+ monocytes were cultured with RANKL and M-CSF in 10% FBS RPMI 1640 medium for 7 days, followed by the addition of anti-CD38 mAb (SAR, 1, 10, 100 μg/mL) into the medium for an additional 7 days. At day 14, CD38 expression on OCs was examined by flow cytometry and immunoblotting. (D-E) CD14+ monocytes were cultured with RANKL/M-CSF in 10% RPMI medium for 7 days, and then SAR (0.1, 1 μg/mL) or IFN-γ (20 IU/mL) were added for an additional 7 days followed by TRAP staining to determine TRAP+ MNC ( >3 nuclei). (F-G) After CD14+ monocytes were stimulated with RANKL and M-CSF for 14 days, SAR, activated autologous T cells (Tact), and IDO inhibitor were added. After 3 days, cytotoxicity was evaluated by measuring LDH activity in supernatants. OCs were stained with Annexin V/PI and analyzed by flow cytometry. MNC, multinucleated cells; PI, propidium iodide.
The impact of anti-CD38 Ab on OC differentiation from CD14-purified monocytes ex vivo. (A) CD38 was examined by immunoblotting in indicated cells. (B) CD38 expression was examined by flow cytometry during osteoclastogenesis. (C) Purified CD14+ monocytes were cultured with RANKL and M-CSF in 10% FBS RPMI 1640 medium for 7 days, followed by the addition of anti-CD38 mAb (SAR, 1, 10, 100 μg/mL) into the medium for an additional 7 days. At day 14, CD38 expression on OCs was examined by flow cytometry and immunoblotting. (D-E) CD14+ monocytes were cultured with RANKL/M-CSF in 10% RPMI medium for 7 days, and then SAR (0.1, 1 μg/mL) or IFN-γ (20 IU/mL) were added for an additional 7 days followed by TRAP staining to determine TRAP+ MNC ( >3 nuclei). (F-G) After CD14+ monocytes were stimulated with RANKL and M-CSF for 14 days, SAR, activated autologous T cells (Tact), and IDO inhibitor were added. After 3 days, cytotoxicity was evaluated by measuring LDH activity in supernatants. OCs were stained with Annexin V/PI and analyzed by flow cytometry. MNC, multinucleated cells; PI, propidium iodide.
Based on these results, we hypothesized that osteoclastogenesis may be strongly inhibited by IFN-γ–producing T cells. We therefore cocultured OCs and activated T cells for 3 days, in the presence of SAR and IDO inhibitor. Cytotoxicity was then performed by measuring LDH activity released into the culture medium. SAR alone inhibited only 5% OCs. In contrast, death of OCs was strongly induced when cocultured with activated T cells. Importantly, SAR enhanced the cytotoxicity of these activated T cells (Figure 6F). These results were further confirmed by Annexin V and PI staining, and flow cytometry (Figure 6G).
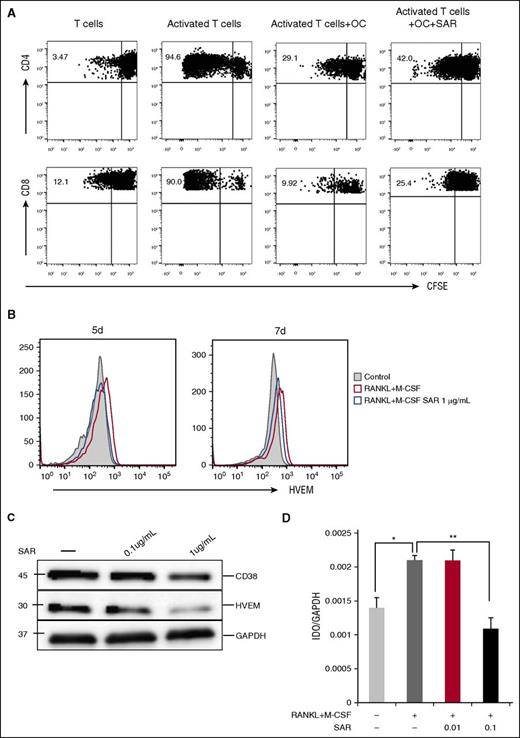
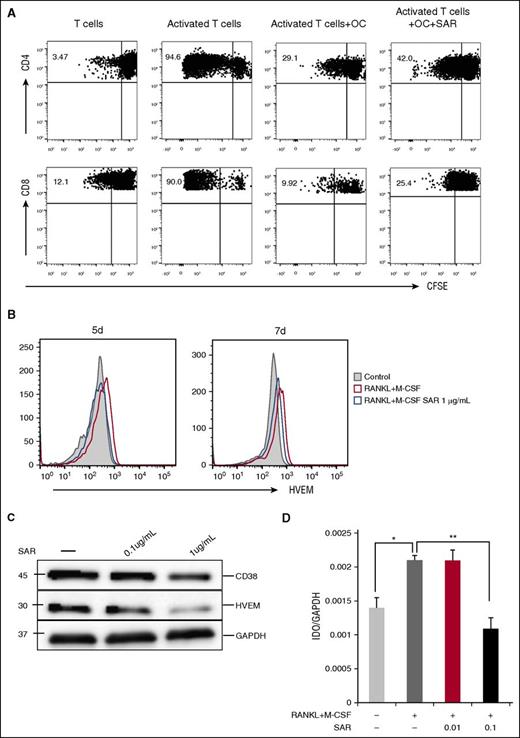
Anti-CD38 mAb restores T-cell response by inhibiting the expression of immune-checkpoint molecules on OCs
Because SAR had minimal effect on osteoclastogenesis derived from CD14+ monocytes in ex vivo cultures and OC formation is mediated by RANKL/IFN-γ–expressing T cells, we next examined whether SAR restores T-cell function. We cultured OCs and T cells from the same donor in vitro for 6 days in the absence or presence of SAR and then assessed T-cell proliferation using CFSE dilution assay. Both CD4 and CD8 T-cell proliferation induced by anti-CD2/CD3/CD28 beads was significantly inhibited in the presence of OCs, whereas OC inhibition on T-cell proliferation was significantly overcome by SAR (Figure 7A). Further, SAR downregulated the expression of HVEM and IDO1 (Figure 7B-D), whereas minimal changes in PD-L1 and Galectin-9 expression were seen (supplemental Figure 2B-C).
Anti-CD38 Ab restores T-cell response. (A) Proliferation of T cells stimulated by anti-CD2/CD3/CD28 beads (T:Bead ratio of 1:1) in the absence or presence of SAR anti-CD38 Ab (1 μg/mL) for 6 days was measured with CFSE dilution assay. (B-D) After CD14+ monocytes were stimulated with recombinant RANKL and M-CSF for 7 days, SAR anti-CD38 Ab was added for 7 days, and cells were examined for HVEM by flow cytometry (B), western blotting (C), and IDO1 by real-time qRT-PCR (D). *P < .05; **P < .001; by unpaired 2-sided Student t test.
Anti-CD38 Ab restores T-cell response. (A) Proliferation of T cells stimulated by anti-CD2/CD3/CD28 beads (T:Bead ratio of 1:1) in the absence or presence of SAR anti-CD38 Ab (1 μg/mL) for 6 days was measured with CFSE dilution assay. (B-D) After CD14+ monocytes were stimulated with recombinant RANKL and M-CSF for 7 days, SAR anti-CD38 Ab was added for 7 days, and cells were examined for HVEM by flow cytometry (B), western blotting (C), and IDO1 by real-time qRT-PCR (D). *P < .05; **P < .001; by unpaired 2-sided Student t test.
Discussion
Monocyte-derived cells are very important for both MM survival and immune escape. They are the major source of APRIL in MM infiltrated BM,32 where macrophages, MDSCs, and DCs mediate immune suppression. Importantly, OCs, also of monocyte-derived lineage, are highly activated in the BM microenvironment to promote proliferation and survival of MM cells.33 In this study, we further identify an immunosuppressive role of OCs in the MM BM microenvironment via 3 mechanisms (supplemental Figure 3): (1) induction of T-cell apoptosis by upregulating immune checkpoint proteins PD-L1, Galectin-9, HVEM, and CD200; (2) induction of IDO and CD38, which regulate T-cell metabolism and function; and (3) production of cytokines, especially APRIL, which further induces PD-L1 in MM cells. Importantly, therapeutic anti-CD38 mAb partially overcame the immunosuppressive effect of OCs associated with decreased HVEM and IDO, thereby restoring the cytotoxic function of T cells.
A previous study suggests that OCs could serve as APCs.13 Recent studies have also demonstrated that OCs express a number of immune receptors, and are regulated like macrophages and DCs.4 In addition, OCs and macrophages shared phenotypic features, such as high numbers of lysosomes. Tumor-associated macrophages in the myeloma microenvironment protect myeloma cells from chemotherapy-induced apoptosis.12 Based on these observations, it would be plausible to hypothesize that OCs in the MM microenvironment may play a similar role as tumor-associated macrophages. We first analyzed the expression of immune-checkpoint molecules on OCs following the observation that OCs inhibit T-cell proliferation. The PD-1/PD-L1 axis is a master immune checkpoint regulating antitumor immune responses against many neoplasms. In addition to high expression of PD-L1 on MDSCs and DCs in the MM microenvironment,10,34 we found high and consistent PD-L1 expression on OCs here. This is further enhanced by IFN-γ. Specially, the expression of PD-L1 is much higher in OCs than in MM cells. PD-L1 expression in OCs, like that in MM cells, could worsen immune inhibition by enhancing binding of PD-1 on T cells. Although blockade of the PD-1 immune checkpoint augmented antitumor immunity and induced durable responses in patients with Hodgkin lymphoma and some solid tumors, PD-1 inhibitors failed to induce significant clinical responses in a phase 1 study in myeloma.35 A recent report suggests that PD-L1 expression by infiltrating cells is more predictive of response to PD-1 pathway blockade than PD-1 expression on the tumor cells.36 Importantly, we show that OCs express PD-L1 to induce immune suppression, thereby our data suggest potential effects of PD-L1 inhibitors in MM.
CD200, another negative immune-checkpoint protein, is expressed in OCs. It is a membrane glycoprotein that mediates an immune regulatory signal through CD200R to suppress T- and natural killer cell-mediated immune responses. CD200 expression on acute myeloid leukemia cells promotes tumor growth in mice via tumor immune evasion involved in CD200/CD200R interaction.37 CD200 expression on MM cells has also been reported to confer worse prognosis.38 Because CD200 expression on OCs is critical for cellular fusion and OC differentiation, the OC number in CD200-deficient mice was decreased, whereas bone mass was increased.8 Conversely, we showed that increased CD200 on OCs as well as on MM cells may induce inhibition of T cells.
HVEM, widely expressed on APCs, endothelial cells, and lymphocytes,39 can also inhibit immune antitumor activity. BTLA protein, an HVEM receptor, is an inhibitory receptor with structural similarities to CTLA-4 and PD-1. Because BTLA is overexpressed on diverse tumor types, this pathway may evolve to evade immune responses. We show here that CD14+ human oligodendrocyte progenitor cells express HVEM, which is increased during osteoclastogenesis. However, the relative importance of HVEM expression on OCs remains to be determined.
IDO is an enzyme that catalyzes the first- and rate-limiting step associated with the catabolic conversion of Trp into kynurenine. T cells require adequate Trp levels for survival and effector function; therefore, IDO-mediated Trp deficiency results in T-cell tolerance and impaired effector function, as well as promotes the differentiation of naive CD4 T cells into regulatory T cells.40 Consistent with a precious study,41 we also show that IDO in OCs mediates the immunosuppression of T cells. IDO was significantly increased in BM plasma of MM patients. However, there was no significant difference in their levels in the serum between healthy individuals and MM patients. It suggests that IDO is hijacked by tumor cells to suppress immune responses by blocking the localized T-lymphocyte proliferation in the BM only, rather than systemically. Expression of IDO is therefore a common mechanism by which MDSCs, DCs, macrophages, and some tumor cells evade T-cell immune suppression, making it an ideal target for cancer immunotherapy.
Galectin-9, a conserved glycan-binding protein, plays various critical roles in both innate and adaptive immunity. During inflammation, the production of Galectin-9 generates a microenvironment limiting effector T-cell responses. The interaction of Galectin-9 with its receptor Tim-3, negatively regulates T helper 1 cell response, resulting in the induction of T-cell apoptosis and exhaustion.21 Here, we observed high expression of cell surface and secreted Galectin-9 in OCs, suggesting that cell surface Galectin-9 may negatively regulate T helper 1 cell response in contact with neighboring T cells. Importantly, our results showed that Galectin-9 impacted T cells but did not directly affect MM cells. Moreover, secreted Galectin-9 may suppress T cells through a noncontact degradation mechanism. Indeed, Galectin-9 levels were high in MM patient BM plasma, consistent with localized immune suppression.
Myelopoiesis dysregulation, characterized by an increased proportion of precursor cells, occurs in MM patients.42,43 These myeloid precursor cells may have the capacity to differentiate into OCPs in the presence of RANKL. OCPs give rise to macrophages, DCs, and OCs depending upon the presence of cytokines and growth factors.44 OCPs show similar function as MDSCs and suppress the in vitro proliferation of CD4+ and CD8+ T cells via the production of nitric oxide.45 In addition, MDSCs are OCPs and differentiate into OCs in the context of a RANKL-rich milieu.46,47 Importantly, the differentiation of OCPs into OCs does not diminish their ability to inhibit T-cell proliferation.45 We here observe that OCPs or immature OCs highly express immune-checkpoint molecules, thereby further conferring immune suppression in MM. It was consistent with the previous study that activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1.20
CD38high MDSCs possess a greater capacity to suppress activated T cells.48,49 CD38 plays a role in OC formation and bone resorption, and functions as a cellular NAD+ sensor.50 Here we observe that CD38 is upregulated during osteoclastogenesis. Anti-CD38 mAb recently showed efficacy as monotherapy in relapse and refractory MM.30 We show here that anti-CD38 mAb significantly decreases CD38 expression in a 2-week ex vivo culture system from purified CD14+ monocytes without impact on OC formation and survival. These results are consistent with a report of CD38−/− mice showing a dramatic increase in OC formation triggered by RANKL and M-CSF, which is associated with markedly reduced bone mineral density.50 Besides, CD38 polymorphism is also associated with premenopausal/postmenopausal bone mineral density and bone loss.51
OCs and osteoclastogenesis were very sensitive to IFN-γ and activated T cells. Activated T cells promote osteoclastogenesis through RANKL expression and they can also negatively affect osteoclastogenesis through IFN-γ production.6 Thus, the balance between RANKL and IFN-γ regulates OC formation. Our data suggests that T-cell exhaustion seems to contribute to bone disease in MM; conversely, restoration of T-cell function may not only improve the efficacy of cancer immunotherapies, but also alleviate bone disease. Indeed, anti-CD38 mAb restores the proliferative activity of CD4 and CD8 T cells partially via downregulating the expression of HVEM and IDO in OCs. Our data therefore indicate that anti-CD38 mAb has limited direct cytotoxicity against OCs and osteoclastogenesis, but may nevertheless enhance immunotherapeutic activity and alleviate bone disease by restoring T-cell function.
In conclusion, our current study characterizes an immunosuppressive role of OCs in MM via upregulating various inhibitory checkpoint molecules and immune-suppressive cytokines. Importantly, OC-induced immunosuppression can be overcome, at least in part, by anti-CD38 mAb. These results further support targeting these checkpoint molecules in combination with anti-CD38 mAb to restore anti-MM immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicholas DaSilva (former employee at DFCI) for his excellent technical assistance, and all clinical and laboratory members of the Jerome Lipper Multiple Myeloma Center of the DFCI for support and help with this study.
This study was supported by grants from the National Institutes of Health (NIH), Research Project Grant Program (RO1050947), NIH National Cancer Institute (PO1-CA078378), Dana-Farber/Harvard Cancer Center/Specialized Programs of Research Excellence in Multiple Myeloma (P50CA100707 [K.C.A.]); and the National Natural Science Fund (81400175, 81670202). K.C.A. is an American Cancer Society Clinical Research Professor.
Authorship
Contribution: G.A. designed and performed all experiments and contributed to the written paper; C.A. designed and performed the experiments; X.F., K.W., M.Z., and L.Z. performed experiments; Y.-T.T. and N.C.M. contributed to the conceptual idea, critical writing, and editing of the paper; and L.Q. and K.C.A. served as principal investigators for the study, and contributed to the conceptual idea for the paper, experimental design, critical suggestions, and writing and editing of the paper.
Conflict-of-interest disclosure: Y.-T.T. is a consultant for Onyx. K.C.A. serves on advisory boards of Onyx, Celgene, Gilead, Bristol-Myers Squibb, and Sanofi-Aventis, and is a scientific founder of Acetylon and Oncopep. N.C.M. is a consultant for Celgene, Onyx, Janssen, and Oncopep, and has an ownership interest in Oncopep. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Boston, MA 02215; e-mail: kenneth_anderson@dfci.harvard.edu; and Lugui Qiu, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Tianjin, China; e-mail: qiulg@ihcams.ac.cn.