To the editor:
Although primary myelofibrosis (PMF) is generally regarded as arising from a mutated stem or progenitor hematopoietic cell, immune dysregulation is common. For example, there are increased plasma levels of inflammatory cytokines and clinical and laboratory manifestations of autoimmunity.1 Regulatory T cells (Tregs) defined as CD4-positive (CD4+) with high expression of CD25 (interleukin-2 receptor) and transcription factor forkhead box P3 (FOXP3) are central to maintaining T-cell homeostasis and immune tolerance.2-7 Abnormalities in Tregs are associated with autoimmune diseases,8-15 and a Treg deficit resulting from a FoxP3 mutation is associated with aggressive autoimmunity and early death.16 There are contradictory data on numbers of Tregs in persons with PMF.17-19
With the aim of determining whether Tregs were abnormal and interrogating possible associations with clinical and laboratory features, we did a cross-sectional evaluation of 202 consecutive subjects with PMF referred to the Center for the Study of Myelofibrosis, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo of Pavia, Italy, from March 2008 to September 2015 (supplemental Methods, available on the Blood Web site). Sample size was defined by investigating blood Treg frequency in 31 subjects with PMF tested twice within 5 days. The coefficient of variation was 1.48 indicating numbers of subjects needed to detect significant differences in group medians was 96 per cohort.20 Thirty-six subjects were analyzed at diagnosis, whereas 166 were analyzed after the diagnosis (median time from diagnosis, 70 months; range, 4-374 months). All subjects were off-therapy at time of sampling. Diagnosis of PMF was confirmed by analyses of bone marrow biopsy samples by an expert pathologist. Patients with documented autoimmune disease or with a previous diagnosis of autoimmune disease were excluded, as were those with concomitant major infections. No patient had received or was receiving JAK2 inhibitor therapy, immunosuppressive therapy, corticosteroids or anti-inflammatory therapies, or interferon, and those studied after hydroxyurea therapy were off-therapy for at least 3 months. Twenty-four healthy subjects matched for age and gender were controls. From 17 additional subjects with PMF splenectomized for a symptomatic splenomegaly, we determined Treg frequency in the spleen samples in order to exclude that the reduced frequency of blood Tregs resulted from a recruitment of these cells in the spleen. Spleen samples from 8 otherwise healthy subjects splenectomized for abdominal trauma were controls. The ethics committee of our institution approved the study, and all subjects gave written informed consent.
To determine whether Tregs from patients with PMF maintained the same antiproliferative activity on effector T cells, we cocultured CD4+CD25+ with CD4+CD25− T cells to determine the ability of Tregs to suppress proliferation of CD4+CD25− effector T cells (supplemental Methods). The percentage of Treg suppression on the proliferation of effector T cells was comparable in patients with PMF (mean, 53.0% ± 23.5% standard deviation) and controls (mean, 48.5% ± 23.2% standard deviation).
Treg frequency was evaluated as CD4+CD25brightCD127lowFOXP3+ cells on peripheral blood or spleen derived mononuclear cells as percentage of CD4+ cells.21 Normal subjects had a median blood Treg frequency of 1.99% (range, 0.48-6.51). In contrast, subjects with PMF had a median Treg frequency of 0.80% (range, 0-10.2; Mann-Whitney U test, P < .001). Normal subjects had a median spleen Treg frequency of 1.20% (range, 0.36-3.97); in contrast, subjects with PMF had a median spleen Treg frequency of 0.35% (range, 0.04-1.26; P < .001). These data suggest the reduced frequency of blood Tregs did not result from a recruitment of these cells in the spleen. Furthermore, the frequency of CD4 peripheral blood cells, evaluated as absolute number per microliter in 40 subjects with MF and 12 normal controls, was comparable (data not shown).
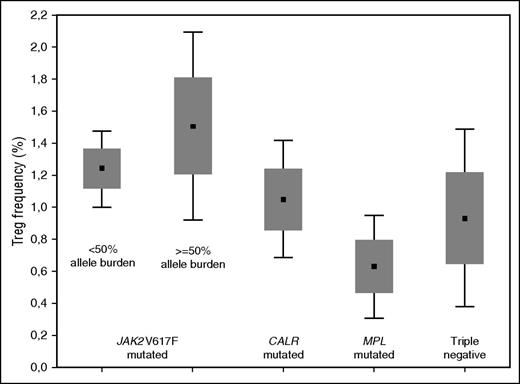
Based on the distribution of Tregs in normal subjects, we established a cutoff point of 0.73% to compare PMF cohorts. Ninety-two subjects with PMF (46%) including 19 subjects at diagnosis (38%) had a decreased frequency of Tregs. There were significant differences in blood Treg frequency between subjects with different PMF genotypes (Figure 1). Treg frequency in subjects with a JAK2V617F genotype (N = 126) was significantly higher than in subjects with a wild-type JAK2 genotype (N = 74, P = .002). This was also so compared with subjects with a CALR mutation genotype (N = 50, P = .06). In subjects with a JAK2V617F genotype, those with an allele burden ≥50% had a higher Treg frequency than those with an allele burden <50% (P = .07). These data suggest that PMF depletes Tregs and that JAK2V617F counters or reverses this effect. The higher frequency of Tregs in subjects with JAK2V617F and an allele burden >50% (even though not statistically significant) is noteworthy and deserves to be confirmed in a greater number of observations. It leads us to hypothesize that the degree of activation of the JAK-STAT pathway influences the Treg level.
Mean values (± standard error and 1.96 standard error) of Tregs in subjects with PMF according to genotype. The difference among Tregs in PMF genotypes was significant (Kruskal-Wallis analysis of variance, P = .05). Treg frequency in persons with a JAK2V617F genotype (N = 126; median, 1.33%; range, 0% to 10.2%) was higher than in subjects with a JAK2 wild-type genotype (N = 74; median, 0.98%; range, 0% to 6.69%; P = .002) and subjects with a CALR mutation genotype (N = 50; median, 1.05%; range 0% to 6.69%; P = .06). Subjects with a JAK2V617F genotype and a ≥50% allele burden had a higher frequency of Tregs than subjects with <50% allele burden (median, 1.50%, range, 0% to 10.2% vs 1.24%, range, 0.01% to 6.11%; P = .07). Subjects with a MPL mutation genotype (N = 9) or no detectable mutation (triple negative) (N = 16) were not included in the comparisons of Treg frequency between PMF genotypes.
Mean values (± standard error and 1.96 standard error) of Tregs in subjects with PMF according to genotype. The difference among Tregs in PMF genotypes was significant (Kruskal-Wallis analysis of variance, P = .05). Treg frequency in persons with a JAK2V617F genotype (N = 126; median, 1.33%; range, 0% to 10.2%) was higher than in subjects with a JAK2 wild-type genotype (N = 74; median, 0.98%; range, 0% to 6.69%; P = .002) and subjects with a CALR mutation genotype (N = 50; median, 1.05%; range 0% to 6.69%; P = .06). Subjects with a JAK2V617F genotype and a ≥50% allele burden had a higher frequency of Tregs than subjects with <50% allele burden (median, 1.50%, range, 0% to 10.2% vs 1.24%, range, 0.01% to 6.11%; P = .07). Subjects with a MPL mutation genotype (N = 9) or no detectable mutation (triple negative) (N = 16) were not included in the comparisons of Treg frequency between PMF genotypes.
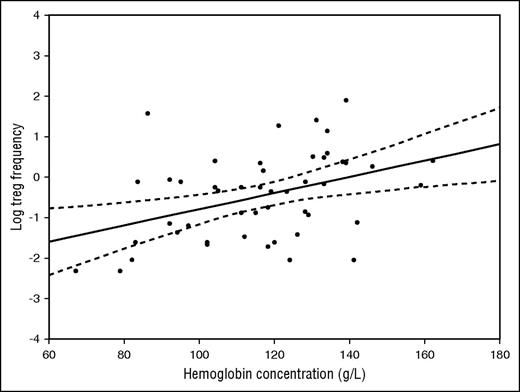
In subjects with and without a JAK2V617F genotype as a whole, there was no significant association between Treg frequency and age, gender, degree of bone marrow fibrosis, International Prognostic Scoring System (IPSS)/ Dynamic IPSS (DIPSS) score (analyses of variance, P > .05), disease duration, spleen or liver size, blood hemoglobin concentration, blood white blood cell count, blood platelets, or CD34+ frequency (Pearson correlation coefficient, P > .05). In contrast, in subjects with a CALR mutation genotype, there was an association between reduced Treg frequency and longer disease duration (R = −0.28, P = .035), increasing age (R = −0.36, P < .007), and higher IPSS/DIPSS score (P = .013). Moreover, there was a strong direct correlation between Treg frequency and hemoglobin concentration (R = 0.50; P < .001; Figure 2). At the time of sampling, none of the CALR-positive patients had had blood transfusions.
Correlation between Tregs (as the natural logarithm of the percent value) and hemoglobin concentration (g/L). Pearson correlation coefficient was 0.50 (P < .001)
Correlation between Tregs (as the natural logarithm of the percent value) and hemoglobin concentration (g/L). Pearson correlation coefficient was 0.50 (P < .001)
Autoimmune contribution to anemia has been previously hypothesized,22 and a causative role of Treg deficiency is sustained by the experimental evidence that disruption of Treg-effector T-cell homeostasis contributes to anemia, and humans with nonfunctional or lack of Tregs develop anemia.16,23
However, anemia and Treg reduction may both portray the response to excess inflammatory cytokines present in PMF.1 To test this hypothesis, we evaluated the circulating levels of serum interleukin-2 receptor α (sIL2Rα) in 93/202 patients with PMF and 10 normal subjects. As previously reported,19,24-26 sIL2Rα blood levels were higher (P = .014) in patients (median, 1154 pg/mL; range, 276-8968) than in normal subjects (median, 778 pg/mL; range, 272-1177). Although patients with Treg frequency >0.73% (n = 56; median, 978 pg/mL; range, 275-4891) were comparable to normal subjects, those with Treg frequency <0.73% had sIL2Rα levels (n = 37; median, 1413 pg/mL; range, 476-8968) higher (P = .004) than normal subjects and inversely correlated to blood Tregs (R = −0.38; P = .009). These data suggest that in patients with PMF, high levels of sIL2Rα are associated with decreased circulating Tregs and are possibly related to the autoimmune phenomena. Similarly, in patients with myeloproliferative neoplasms with no clinical overt evidence of autoimmune diseases, those with at least 1 positive autoimmune serology have significantly elevated sIL2Rα blood levels than those with negative serology.19
In conclusion, a low frequency of circulating Tregs, a nonclonal component of bone marrow and spleen microenvironment, characterizes patients with PMF. We found that decrease in blood Treg frequency was greatest in subjects with a wild-type JAK2 genotype, and those with a CALR mutation had a Treg frequency significantly associated with advanced disease, higher IPSS/DIPSS score, and lower hemoglobin concentration. Moreover, we suggested a role of sIL2Rα in hampering the physiological expansion of Tregs.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy) “Special Program Molecular Clinical Oncology 5x1000” to AGIMM (AIRC–Gruppo Italiano Malattie Mieloproliferative). A detailed description of the AGIMM project is available at http://www.progettoagimm.it.
Contribution: M.M., R.C., G.B., and V.R. designed the study, collected data from patients in Pavia, analyzed the results, and wrote the manuscript; G.F. helped with the Treg assay; V.P. helped with collection of clinical information; L.V. helped with the JAK2V617F, CALR, and MPL mutation detection; E.B. helped with collection of clinical information; G.V. helped with the assay of CD34+ cells; P.C. helped with the JAK2V617F assay with quantitative polymerase chain reaction; M.D.A. helped with the assay of sIL2Rα; and R.P.G. helped with manuscript writing and data interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni Barosi, Center for the Study of Myelofibrosis, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: barosig@smatteo.pv.it.