To the editor:
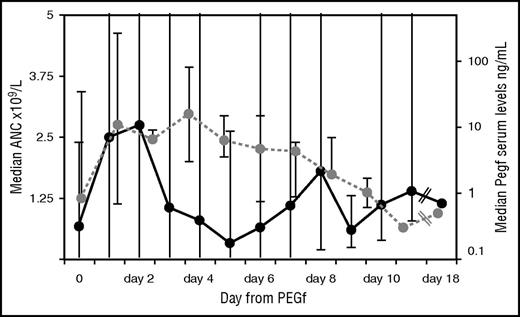
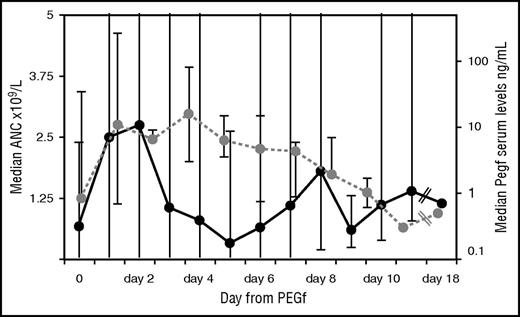
Severe congenital neutropenia (SCN) is a rare disease characterized by a severe defect in neutrophil production and by high risks of lethal infection.1,2 Lifetime treatment with granulocyte colony-stimulating factor (G-CSF) is indicated in patients responding to standard doses (up to 5 μg/kg per day). In those requiring higher doses of G-CSF (>8 μg/kg per day) or those who have transformed into myelodysplasia acute myeloid leukemia (MDS/AML), hematopoietic stem cell transplantation might be considered, especially if an excellent HLA-matched donor is available.3-5. Subcutaneous daily injection of G-CSF may seriously limit treatment compliance, thus increasing the risk of infections, particularly in the youngest children. Because of its long half-life, the pegylated form of filgrastim, pegfilgrastim (PEGf), would enable significant reduction in the frequency of injections, thus improving adherence to therapy and infection control. This drug is currently used in patients with solid tumors to shorten the duration of neutropenia and in the autotransplant setting to mobilize stem cells.6-8 The use of PEGf in SCN has been reported only in single patients and in retrospective cohorts with limited pharmacokinetic analysis.9-12 One limitation might derive from severe skin and lung toxicities, which were mainly observed in patients with cyclic neutropenia or glycogen storage disease type Ib but not in classical SCN.9 In this study, we describe the long-term outcome of PEGf treatment in children with SCN who were poorly compliant to classical G-CSF (filgrastim). Five patients entered this study (registered at the Observatory of the Italian Ministry of Health, Eudract Code 2005-003096-20) after informed consent was obtained from the parents. Two children were already described, with considerably shorter follow-up.10 After 72-hour washout from filgrastim, subcutaneous PEGf was initially administered at a dose of 100 μg/kg at an interval not shorter than 4 days. Subsequent injections aimed to maintain median absolute neutrophil count (ANC) at 1 × 109 to 5 × 109/L and/or to control infection. Bone marrow morphology, cytogenetics, granulocyte-colony stimulating factor receptor (G-CSFR) mutation analysis, abdominal ultrasound scan, and bone density assessment by dual energy radiograph absorptiometry were performed at baseline and then yearly. Blood count, biochemistry, and serum concentration of PEGf (enzyme-linked immunosorbent assay, Quantikine HS; R&D System Inc., MN) were frequently evaluated within the first 6 months and then again 3 to 4 times per year. Quality of life was evaluated using the Short Form Health Survey questionnaire (SF-36) by the parents at the beginning and end of follow-up.13 Infections were quantified by infectious ratio (IR), which considers the number of documented infections supported by clinical, imaging, biochemical, and/or microbiological findings over the period at risk/patient, normalized by 1000 days.14 From July 2006 to October 2015, 5 consecutive patients (3 males) diagnosed with SCN (4 mutated in ELANE and 1 in HAX-1 genes) at a median age of 2 months (0-18 months) were enrolled in the study. Characteristics of the cohort are shown in Table 1. Before PEGf, 4/5 patients were treated with daily subcutaneous G-CSF, to which they were poorly compliant, for a median of 36 months (0.23-89 months). Filgrastim median dose was 7.5 μg/kg (5-25 μg/d). The initial PEGf schedule was 50 to 100 μg/kg every 7 to 12 days to reach the ANC between 1 × 109 and 5 × 109/L. Median age at start of PEGf was 50 months (7-110 months). Median follow-up was 46 months (7-111 months). PEGf increased neutrophils in 4/5 patients. Median ANC of the cohort was 1.5 × 109/L (0 to 34 × 109/L), which is higher, although not significantly, than that on filgrastim (1 × 109/L). Median IR was 5.1 (3.7-6.3), which is lower, although not significantly, than that on filgrastim. However, when PEGf was given every 7 to 8 days, median ANC was significantly higher (1.28 × 109/L) than that achieved by administration every 9 to 12 days (0.67 × 109/L; P = .002, Kruskal-Wallis test). Median IR was significantly lower with the 7- to 8-day schedule (4.5) as compared with the 9- to 12-day schedule (6.3; P = .029, Kruskal-Wallis test). The ANC (Figure 1), shows a bimodal pattern with a first ANC peak 24 to 48 hours after PEGf administration, a decline below 1 × 109/L after 2 to 5 days, and a second rise on days 6 to 7 without any further drug administration, followed by a second drop. Peak serum concentration of PEGf was achieved 24 to 72 hours after administration, and then levels declined to those of the washout phase (pre-PEGf) within day 9. PEGf serum concentrations of day 0 were comparable to those measured at the same time point (before drug injection) during filgrastim treatment and did not increase over the yearly follow-up. Compliance and quality of life measured through the SF-36 questionnaire showed a global amelioration because of reduced physical and mental limitation in turn attributable to better control of body pain (P = not significant). Neither local nor generalized reactions occurred during PEGf. No cytogenetic abnormalities were documented. In 1 patient, 2 G-CSFR mutations (c.2384C>T and c.2425T>G) and progression to osteoporosis were documented (after 24 months of G-CSF and 46/40 months of PEGf treatment). Renal and liver function tests remained normal. No organ toxicity was reported over the entire follow-up. Although in a small-sized cohort, this is the first comprehensive prospective analysis with the longest ever reported follow-up (maximum 9 years) of the outcome of PEGf treatment in SCN patients. This study shows that PEGf allowed the median ANC count to rise above 1.5 × 109/L and to reduce infections in comparison with the previous filgrastim phase. Benefits (increased ANC and reduced IR) were more clearly evident (ie, statistically significant) as compared with the prior filgrastim phase, when PEGf was given every 7 to 8 days vs every 9 to 12 days. Infections were not fully cleared during PEGf despite the increased neutrophil count probably because of the lack of full rescue of neutrophil function. However, the catch-up growth seen in 2 patients during the PEGf compared with the G-CSF period could be an indirect effect of better infection control. Overall, PEGf was safe because no acute/chronic side effects were reported, with the exception of 1 patient who developed osteoporosis and after a few months acquired a G-CSFR mutation. Because of prior G-CSF treatment, we cannot establish the exact role of PEGf in these events. In addition, these effects may not be attributed unequivocally to therapy because of the intrinsic tendency of the disease itself toward osteoporosis and clonal evolution that may be exacerbated by G-CSF. Transformation to MDS/AML occurs at variable rates of 11% to 31% after 10 to 15 years of G-CSF therapy, and it is dose dependent.15-18 Peak serum concentrations of PEGf were comparable to those measured in solid tumor patients and did not increase over time. Moreover, the lowest levels of G-CSF were similar during filgrastim and PEGf, thus excluding the risk of overexposure of the stem cells as compared with the previous filgrastim phase. Regarding the costs, at the prices the company offered to the pharmacy of our institute at the time of protocol initiation, a daily dose of 5 μg/kg of filgrastim was equivalent to that of a full vial of PEGf (including discarded amount) given every 8 days, an interval of administration that significantly reduced IR, thus pointing to an additional indirect cost advantage.
ANC and pharmacokinetic (PK) in the whole cohort. Black line represents the ANC trend at given days after a single injection of PEGf over the entire follow-up in the whole group; dots are median values of ANC; and the bars correspond to the maximum/minimum for each day after PEGf inoculum. Likewise for PK (dotted gray line); dots indicate median PK values observed at a given day after a single PEGf injection, and the bars correspond to minimum/maximum for the days when available.
ANC and pharmacokinetic (PK) in the whole cohort. Black line represents the ANC trend at given days after a single injection of PEGf over the entire follow-up in the whole group; dots are median values of ANC; and the bars correspond to the maximum/minimum for each day after PEGf inoculum. Likewise for PK (dotted gray line); dots indicate median PK values observed at a given day after a single PEGf injection, and the bars correspond to minimum/maximum for the days when available.
In conclusion, this analysis, even if it includes a limited number of patients, is rather extensive and shows that PEGf may be a beneficial alternative in patients poorly compliant to classical G-CSF, because, during long-term follow-up, it increases neutrophils, reduces IR, and improves quality of life at a cost of drug exposure similar to that for classical G-CSF. Increasing the number of patients and prolonging follow-up will provide further information on the risk of clonal evolution and on the role of PEGf vs filgrastim in the management of SCN patients.
Authorship
Acknowledgments: Edoardo Raffinerie Garrone S.p.A., Genova; Rimorchiatori Riuniti, Genova; Cambiaso & Risso Marine, Genova; and Saar Depositi Oleari Portuali, Genova, are acknowledged for supporting the activity of the Haematology Unit of IRCCS Giannina Gaslini, Genova. Valentina Faccio is acknowledged for secretarial support and management of the study.
Contribution: C.D., F.F., and M.C. conceived and designed the study and wrote the manuscript; S.S., F.G., and E.M. collected the materials; S.Z. performed the statistical analysis; M.L. and T.L. performed PK analysis and molecular studies; and F.R. analyzed physiological aspects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesca Fioredda, IRCCS Giannina Gaslini, Children’s Hospital, Via Gerolamo Gaslini 5, 16147 Genova, Italy; e-mail: francescafioredda@gaslini.org.