Abstract
Light chain (AL) amyloidosis is caused by a usually small plasma cell clone producing a misfolded light chain that deposits in tissues. Survival is mostly determined by the severity of heart involvement. Recent studies are clarifying the mechanisms of cardiac damage, pointing to a toxic effect of amyloidogenic light chains and offering new potential therapeutic targets. The diagnosis requires adequate technology, available at referral centers, for amyloid typing. Late diagnosis results in approximately 30% of patients presenting with advanced, irreversible organ involvement and dying in a few months despite modern treatments. The availability of accurate biomarkers of clonal and organ disease is reshaping the approach to patients with AL amyloidosis. Screening of early organ damage based on biomarkers can help identify patients with monoclonal gammopathy of undetermined significance who are developing AL amyloidosis before they become symptomatic. Staging systems and response assessment based on biomarkers facilitate the design and conduction of clinical trials, guide the therapeutic strategy, and allow the timely identification of refractory patients to be switched to rescue therapy. Treatment should be risk-adapted. Recent studies are linking specific characteristics of the plasma cell clone to response to different types of treatment, moving toward patient-tailored therapy. In addition, novel anti-amyloid treatments are being developed that might be combined with anti-plasma cell chemotherapy.
Introduction
Amyloidoses are protein conformational diseases caused by misfolding and aggregation of autologous proteins that deposit in tissues in the form of amyloid fibrils. More than 30 different proteins have been identified as possible causes of amyloidosis, and mass spectrometry-based diagnostics are constantly increasing this number. Amyloid deposition can be systemic or localized, such as in cerebral amyloidoses (the most frequent being Alzheimer disease) and in localized light chain (AL) amyloidosis, mostly involving the airways, skin, and urinary tract, which usually does not require systemic therapy.1,2 The most common forms of systemic amyloidoses are listed in Table 1. AL amyloidosis accounts for more than three-fourths of patients and is caused by a plasma cell clone that in approximately 50% of cases infiltrates the bone marrow by less than 10%. This is the most frequent of a large group of rare diseases characterized by organ damage caused by the monoclonal protein produced by small dangerous plasma cell clones.3 These diseases can target several organs, and those involving the kidney have recently been grouped in the category of monoclonal gammopathies of renal significance.4 In the last decade, improvements in the understanding of the pathogenesis of organ damage were accompanied by the availability of accurate biomarkers of clonal and organ disease and by the development of novel therapeutic agents. This resulted in a rapidly changing approach to patients with improvement of long-term survival over time, as reported by our group and others.5-7 In recent series, 4-year overall survival ranges from 40% to 60%. However, no improvement was observed in early mortality resulting from advanced cardiac damage, with approximately 30% of patients dying within 1 year from diagnosis.
Diagnosis
Several organs can be involved by AL amyloidosis (Table 1), and clinical presentation and outcome depend on the pattern and severity of organ involvement. The clinical manifestations of systemic AL amyloidosis are protean and are usually a consequence of advanced organ damage, mimicking other more common conditions of the elderly. Thus, although combinations such as heart failure and nephrotic syndrome or “left ventricular hypertrophy” on echocardiography without consistent electrocardiographic evidence should raise suspicion of this disease, AL amyloidosis is often diagnosed late. A recent study revealed that in almost 40% of cases, the disease is diagnosed more than 1 year after the onset of symptoms.8 This delay explains the high proportion (∼30%) of subjects who present with advanced, irreversible organ damage and die within 12 months from diagnosis, despite improvement of treatment approaches over time.9,10 The availability of biomarkers of presymptomatic organ damage, N-terminal pro-natriuretic peptide type-B (NT-proBNP), with 100% diagnostic sensitivity in cardiac AL amyloidosis,11 and albuminuria for renal involvement, prompted us to advocate a biomarker-based screening in patients with monoclonal gammopathy of undetermined significance and abnormal free light chain (FLC) ratio who are at risk of developing AL amyloidosis, aiming at reducing late diagnoses and improving survival.9,12
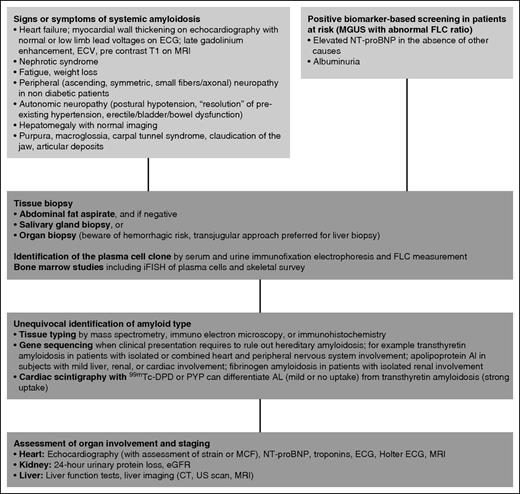
The conditions leading to suspect systemic amyloidosis and the procedures required for diagnosis are summarized in Figure 1. The diagnosis requires the demonstration of amyloid deposits in a tissue biopsy. With an 81% diagnostic sensitivity in AL amyloidosis,13 abdominal fat is the most easily accessible biopsy site and can be innocuously aspirated. In case of a strong clinical suspicion, biopsies should be obtained at additional sites in patients with negative fat aspirate. The biopsy of a minor salivary gland can also be easily obtained and can identify almost 60% of patients with systemic amyloidosis and negative abdominal fat.14 If necessary, the involved organ can be biopsied after careful assessment of hemostasis. Biopsy of the amyloid-loaded liver may result in fatal bleeding, and the transjugular route is recommended.
Diagnostic workup of systemic AL amyloidosis. Systemic amyloidosis can be suspected on the basis of symptoms of organ involvement or during biomarker-based follow-up of monoclonal gammopathy of undetermined significance. Imaging is crucial in identifying heart involvement. The echocardiographic features of advanced cardiac amyloidosis are distinctive, with nondilated ventricles showing thickening of ventricular walls, as well as of interventricular and interatrial septa; amyloid infiltration gives a characteristic “granular sparkling” aspect to the myocardial texture. Cardiac magnetic resonance imaging shows global subendocardial late gadolinium enhancement and associated abnormal myocardial and blood-pool gadolinium kinetics. Equilibrium contrast magnetic resonance imaging allows quantification of the myocardial extracellular volume, which is related to amyloid load. Diagnosis is based on tissue biopsy. Less-invasive biopsy sites (abdominal fat, minor salivary glands) can be preferred. Amyloid deposits need to be characterized by reliable techniques to unequivocally identify amyloid type. Staging of organ dysfunction and characterization of the plasma cell clone offer guidance to the design of the therapeutic approach. CT, computed tomography; ECG, electrocardiogram; ECV, extracellular volume; DPD, 3,3-diphosphono-1,2-propanodicarboxylic acid; iFISH, immunofluorescence in situ hybridization; MGUS, monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging; PYP, pyrophosphate; US, ultrasound.
Diagnostic workup of systemic AL amyloidosis. Systemic amyloidosis can be suspected on the basis of symptoms of organ involvement or during biomarker-based follow-up of monoclonal gammopathy of undetermined significance. Imaging is crucial in identifying heart involvement. The echocardiographic features of advanced cardiac amyloidosis are distinctive, with nondilated ventricles showing thickening of ventricular walls, as well as of interventricular and interatrial septa; amyloid infiltration gives a characteristic “granular sparkling” aspect to the myocardial texture. Cardiac magnetic resonance imaging shows global subendocardial late gadolinium enhancement and associated abnormal myocardial and blood-pool gadolinium kinetics. Equilibrium contrast magnetic resonance imaging allows quantification of the myocardial extracellular volume, which is related to amyloid load. Diagnosis is based on tissue biopsy. Less-invasive biopsy sites (abdominal fat, minor salivary glands) can be preferred. Amyloid deposits need to be characterized by reliable techniques to unequivocally identify amyloid type. Staging of organ dysfunction and characterization of the plasma cell clone offer guidance to the design of the therapeutic approach. CT, computed tomography; ECG, electrocardiogram; ECV, extracellular volume; DPD, 3,3-diphosphono-1,2-propanodicarboxylic acid; iFISH, immunofluorescence in situ hybridization; MGUS, monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging; PYP, pyrophosphate; US, ultrasound.
Common types of systemic amyloidoses have overlapping clinical presentations (Table 1), but require radically different treatments, and non-AL amyloidoses need to be unequivocally ruled out before starting anti-plasma cell chemotherapy. Novel treatment approaches have been recently developed for ATTR, the most common form of systemic non-AL amyloidosis, such as TTR tetramer stabilizers, gene silencing by small interfering RNAs and antisense oligonucleotides, small molecules (eg, doxycycline, epigallocatechin gallate) interfering with aggregation and targeting the fibrils, and anti-amyloid antibodies.15 The availability of these new agents makes it even more important to accurately and unequivocally characterize the amyloid deposits to address patients to appropriate specific treatment and avoid inadequate and potentially harmful therapy. However, in the presence of a plasma cell clone in patients with a clinical presentation strongly suggesting AL amyloidosis (subjects with concomitant proteinuria and heart involvement or with periorbital purpura and/or macroglossia), when prompt intervention is needed, it is reasonable to start treatment pending the results of tissue typing. The characterization of amyloid deposits requires adequate technology and expertise, and patients should be referred to specialized centers. Light microscopy immunohistochemistry with commercial antibodies lacks specificity,16 but can correctly classify almost 95% of patients when performed with custom-made antibodies at highly specialized centers.17 Commercial antibodies can be used in immunoelectron microscopy. This technique can achieve 100% specificity and can correctly classify more than 99% of patients with systemic amyloidosis.13 Being not antibody-dependent, mass spectrometry-based proteomics can overcome the limitations of light microscopy immunohistochemistry, greatly improving the diagnostic accuracy.18 Mass spectrometry diagnostics can be performed after laser capture microdissection of Congo red-positive areas from slides obtained from paraffin-embedded tissue19 or on protein extracted from the whole sample.20 Gene sequencing is needed to rule out or confirm possible hereditary amyloidoses. Cardiac scintigraphy with bone tracers (99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid or pyrophosphate) can differentiate AL (mild or no uptake) from transthyretin amyloidosis (strong uptake) and can spare cardiac biopsy, particularly in elderly men with ATTRwt (formerly senile systemic amyloidosis).21
Because of the small size of the plasma cell clone, the identification of the amyloidogenic light chains requires the combination of immunofixation of both serum and urine and measurement of FLCs.22-24 Promising high-resolution mass spectrometry methods to identify and quantify monoclonal light chains are being developed that will flank current tools in the diagnosis and follow-up.25 Bone marrow biopsies should be obtained, and immunofluorescence in situ hybridization of plasma cells might offer guidance to the therapeutic approach.
Prognostic stratification and risk assessment
Heart involvement has the greatest effect on survival, and major advances have been made in understanding the mechanism of cardiac damage. Recent evidence points to a cardiotoxic effect of the circulating precursor. The infusion of light chains from patients with cardiac involvement increases, in a matter of minutes, end-diastolic pressure in isolated mouse hearts.26 The processes through which extracellular light chains lead to cardiac cell pathology are still under investigation, although increased apoptosis, oxidative stress, altered calcium handling, and activation of specific signal transduction pathways have been reported.27,28 Moreover, in patients in whom chemotherapy reduces the concentration of the amyloidogenic circulating light chain, cardiac dysfunction improves, despite the amyloid load remaining unaltered.11,29 Animal models showed that light chains from patients with cardiac involvement cause a reduction of cardiac output and early mortality in zebrafish28 and reduce the pumping rate of Caenorhabditis elegans’ pharynx, an ortholog of vertebrates’ heart with autonomous contractile activity, reminiscent of cardiac myocytes.30 An interaction of amyloidogenic light chains with mitochondrial proteins in cardiac cells, resulting in mitochondrial dysfunction and damage, has been proven.31 Recently, it has been suggested that amyloid fibrils can also impair the metabolism of cardiomyocytes.32 Amyloidogenic light chains cause oxidative stress, and eventually cell death, via a p38 mitogen-activated protein kinase signaling cascade.33,34 The fact that a p38 mitogen-activated protein kinase-dependent pathway also promotes the synthesis of BNP35 provides a robust pathogenic background to the clinical use of BNP and the N-terminal portion of its pro-hormone (NT-proBNP) as markers of cardiac dysfunction in this disease.
The current validated staging systems for AL amyloidosis are reported in Table 2. Patients’ survival is extremely heterogeneous: patients without heart involvement can survive many years even if they do not respond to treatment, whereas most patients with advanced cardiac damage die in a matter of few weeks. Kidney involvement does not have a major effect on survival, but limits quality of life and access to effective treatments. We have recently validated a staging system for renal involvement, based on estimated glomerular filtration rate (eGFR) and proteinuria, that is able to predict the risk for dialysis.36 The cardiac biomarkers NT-proBNP and troponins are powerful predictors of survival. They are combined in a simple, yet accurate staging system that is still the most widely used system for individual patient management and stratification in clinical trials.37 Among stage III subjects, high concentrations of NT-proBNP (>8500 ng/L) or hypotension identify patients with very advanced disease and poor outcome, with most of them dying within a few weeks from diagnosis.38 A limitation of NT-proBNP-based staging systems is the effect of renal failure on the concentration of this biomarker. This interference can be partly overcome by using BNP in subjects with low eGFR (<30 mL/min per 1.73 m2).39 A specific staging system combining liver involvement and neuropathy has been designed for AL amyloidosis caused by immunoglobulin M-producing clones, a distinct clinical entity characterized by less common cardiac and more frequent peripheral nervous system involvement.40 High-sensitivity cardiac troponin T is the single most powerful biomarker predicting survival, and a staging system based on it alone has been proposed and is waiting for validation.41-43 The Mayo Clinic group recently reported that soluble suppression of tumorigenicity 2 predicts survival independent of other biomarkers.44 Growth differentiation factor 15 is also a promising marker of early death and renal outcomes and is currently being studied.45 Cardiac imaging contributes to prognostication of survival. In particular, echocardiographic left ventricular strain improves the predictive value of cardiac biomarkers.46 More recently, myocardial contraction fraction, a simple index of myocardial shortening obtained from standard echocardiography and strongly correlated with left ventricular strain, has been proposed as a new independent prognostic factor.47
Although the severity of heart involvement predicts most early deaths, within the first year after diagnosis, the biology and burden of the plasma cell clone affect treatment outcomes and long-term survival. The difference between involved (amyloidogenic) and uninvolved FLCs (dFLC) is prognostic and can be integrated in the staging system based on cardiac biomarkers.48 Recent observations from our group suggest that patients with high dFLC burden have better outcome when treated with melphalan, dexamethasone, and bortezomib (BMDex) compared with melphalan dexamethasone (MDex) or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).49 The Mayo Clinic investigators showed that patients with a bone marrow plasma cell infiltrate higher than 10% have a poor outcome independent of the presence of overt multiple myeloma.50 Their recent data suggest that these patients are those who benefit most from induction treatment before autologous stem cell transplant (ASCT).51 The Heidelberg group identified immunofluorescence in situ hybridization abnormalities that are associated with treatment outcomes: patients with gain of chromosome 1q21 have poorer outcome when treated with MDex, whereas translocation t(11;14) is associated with inferior survival in patients receiving CyBorD.52,53 Taken together, if confirmed in independent studies, these data could be useful to guide the choice of treatment, moving toward a patient-tailored approach.
Treatment
The treatment of AL amyloidosis has been solely based on anti-plasma cell chemotherapy for many years. By suppressing the plasma cell clone, chemotherapy reduces the concentration of toxic light chains, which is necessary to improve organ dysfunction and prolong survival. More recently, different approaches targeting the amyloid deposits and interfering with organ damage have been developed that are being tested in clinical trials. They may represent powerful complements to chemotherapy.
Chemotherapy
Chemotherapy of AL amyloidosis is based on regimens developed for multiple myeloma. However, AL amyloidosis is not merely a hematologic malignancy, and amyloid-related dysfunction of 1 or more organs not only determines survival but also limits the access of patients to aggressive treatments. This requires a risk-adapted approach, with dose reductions and schedule modifications of chemotherapy regimens and a close monitoring of hematologic and organ response. Only a few controlled studies have been performed in AL amyloidosis, and no prospective randomized trials of novel agents have been published so far. Thus, most of the available data derive from retrospective case series, and results are difficult to compare, given the extreme heterogeneity of this disease (Table 3). For this reason, whenever possible, patients with AL amyloidosis should be treated within clinical trials. Treatment indications are reported in Figure 2.
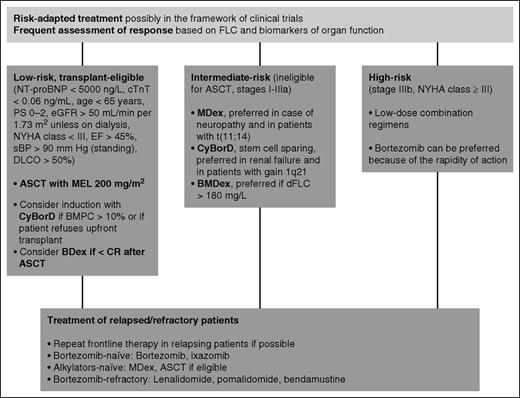
Therapeutic approach to systemic AL amyloidosis. Indications on the therapeutic approach to AL amyloidosis mostly derive from uncontrolled studies. Treatment should be risk adapted. At our center, 14% of patients are low risk and transplant eligible, 42% are intermediate risk, and 44% are high risk. Of the potential ASCT candidates, 80% receive frontline CyBorD. Patients with more than 10% bone marrow plasma cell infiltrate benefit most from induction before ASCT. Post-transplant treatment with bortezomib increases the rate of CR. Characteristics of the amyloidogenic plasma cell clone can guide the choice of chemotherapy: Patients with t(11;14) have a poorer outcome with bortezomib-based therapy, whereas MDex had a worse performance in subjects with gain of 1q21, and BMDex seems superior to MDex and CyBorD in subjects with elevated (>180 mg/L) dFLC. High-risk patients do not tolerate full-dose therapy. These patients should receive low-dose combinations. Low-dose weekly (0.7-1.0 mg/m2) bortezomib is preferred in this setting because of its rapid action. Young patients with isolated advanced cardiac involvement can be considered for heart transplant followed by ASCT. BDex, bortezomib dexamethasone; BMPC, bone marrow plasma cell; DLCO, lung diffusion of CO; EF, ejection fraction; MEL, melphalan; NYHA, New York Heart Association; PS, performance status by Eastern Cooperative Oncology Group; sBP, systolic blood pressure. Stage is standard Mayo Clinic cardiac stage. Stage IIIb is defined as stage III with NT-proBNP level higher than 8500 ng/L.
Therapeutic approach to systemic AL amyloidosis. Indications on the therapeutic approach to AL amyloidosis mostly derive from uncontrolled studies. Treatment should be risk adapted. At our center, 14% of patients are low risk and transplant eligible, 42% are intermediate risk, and 44% are high risk. Of the potential ASCT candidates, 80% receive frontline CyBorD. Patients with more than 10% bone marrow plasma cell infiltrate benefit most from induction before ASCT. Post-transplant treatment with bortezomib increases the rate of CR. Characteristics of the amyloidogenic plasma cell clone can guide the choice of chemotherapy: Patients with t(11;14) have a poorer outcome with bortezomib-based therapy, whereas MDex had a worse performance in subjects with gain of 1q21, and BMDex seems superior to MDex and CyBorD in subjects with elevated (>180 mg/L) dFLC. High-risk patients do not tolerate full-dose therapy. These patients should receive low-dose combinations. Low-dose weekly (0.7-1.0 mg/m2) bortezomib is preferred in this setting because of its rapid action. Young patients with isolated advanced cardiac involvement can be considered for heart transplant followed by ASCT. BDex, bortezomib dexamethasone; BMPC, bone marrow plasma cell; DLCO, lung diffusion of CO; EF, ejection fraction; MEL, melphalan; NYHA, New York Heart Association; PS, performance status by Eastern Cooperative Oncology Group; sBP, systolic blood pressure. Stage is standard Mayo Clinic cardiac stage. Stage IIIb is defined as stage III with NT-proBNP level higher than 8500 ng/L.
A huge international effort has established and validated criteria for assessment of hematologic, cardiac, and renal response to treatment that have been validated in independent series based on patients’ outcomes (Table 4).36,54 These criteria allow the timely identification of refractory patients and can be used as surrogate endpoints in clinical trials, allowing earlier study completion.55 Response should be assessed at least every 2 cycles or 3 months after ASCT, measuring FLC and biomarkers of organ dysfunction. Patients who fail to rapidly achieve very good partial or complete response or organ response should be immediately switched to potentially effective second-line treatment.
The introduction of ASCT represented a major step forward in the treatment of AL amyloidosis.56 However, inappropriate patient selection was responsible for unacceptable transplant-related mortality (TRM), and it soon became clear that the majority of patients are too fragile to undergo ASCT. Refinement of eligibility criteria over time resulted in a progressive reduction of TRM to as low as 5%.57 However, this improvement was limited to patients without cardiac involvement.57 Cardiac biomarkers play a central role in the assessment of eligibility for ASCT. Almost all early deaths occur in patients with cardiac troponin T levels higher than 0.06 ng/mL or NT-proBNP levels higher than 5000 ng/L, who should not be considered candidates for ASCT.58 Other eligibility criteria for ASCT used at our center are reported in Figure 2.9 Similar criteria have been proposed by the Mayo Clinic group.59 A reduction of the dose of melphalan does not significantly reduce TRM, but is associated with response rates lower than those achievable with less toxic regimens.60 Center experience also affects TRM, which is significantly higher (7% vs 3%) at institutions where fewer than 4 transplants are performed every year.57 The hematologic response rate to ASCT exceeds 70%, with 35% to 37% of patients obtaining CR.57,61 The Boston University investigators recently updated their experience with ASCT.61 With a median follow-up of 8 years, the overall median survival was 7.6 years. Remarkably, about 55% of patients in CR are projected to be alive at 14 years, with no deaths observed in patients with longer follow-up.61 This observation raises the hope that a proportion of patients achieving CR according to the current definition might be cured. Bortezomib can be used in subjects who fail to achieve CR after ASCT, increasing the CR rate to almost 60%.62
However, the majority of patients with AL amyloidosis are not eligible for ASCT. At our center, the standard treatment of intermediate-risk patients has been oral MDex. We have recently updated our experience with this regimen.63 With a median follow-up of 6 years, in patients receiving full-dose dexamethasone, 30% of whom were cardiac stage I, 60% stage II or IIIa, and 10% stage IIIb, the median overall survival was 7.3 years. The hematologic response rate was 76%, with 31% of patients obtaining CR.63 These results are comparable to that observed with ASCT. The projected survival of patients in CR after MDex is more than 80% at 7 years.63 However, data on very long term outcome are still lacking. One of the few published randomized trials in AL amyloidosis compared ASCT and MDex.64 This trial has been criticized because of the very high (24%) TRM that was ascribed to suboptimal selection of transplant candidates in an era preceding the use of cardiac biomarkers. Nevertheless, a landmark analysis excluding early deaths failed to demonstrate a survival advantage of one treatment over the other.64 The availability of bortezomib raised great expectation because proteasome inhibitors were thought to be targeted therapy for amyloidogenic plasma cells relying on the proteasome to cope with the proteotoxicity imposed by the misfolded light chain.65 Prospective trials and large retrospective series proved the efficacy and tolerability of bortezomib in AL amyloidosis.66,67 More recently, 2 retrospective series showed unprecedented hematologic response rates (up to 90%, with 60%-65% CRs) in treatment-naive patients receiving CyBorD.68,69 Subsequently, 2 retrospective matched case-control studies confirmed higher response rates with regimens combining bortezomib, dexamethasone, and alkylating agents (BMDex and CyBorD) compared with standard MDex or cyclophosphamide/thalidomide/dexamethasone (CTD), but failed to demonstrate an overall survival advantage.70,71 An international, randomized phase 3 study comparing MDex and BMDex will be completed next year (NCT01277016). An interim analysis showed a higher hematologic response rate with BMDex (76% vs 54%; P = .04), which has not translated into a survival advantage so far.72 We recently published the largest series of patients treated with CyBorD.73 The overall response rate was 60%, and 23% of patients attained CR. Response rates decreased with increasing cardiac stage (Table 3) as a result of early deaths and because of the unfeasibility of full-dose treatment.73
So far, no treatment approach has been able to improve the overall outcome of patients with very advanced cardiac involvement (Table 3). However, even these unfortunate subjects can enjoy a prolonged survival if they respond to treatment, as observed in approximately 20% of patients.73 These subjects do not tolerate high doses of dexamethasone and bortezomib, and should be treated with low-dose combination regimens carefully increasing drug dosages on a week-by-week basis.
Immune-modulatory drugs (IMiDs) have found their place in rescue treatment of patients refractory to upfront regimens or those who relapse but cannot repeat frontline therapy. Lenalidomide and pomalidomide proved able to overcome resistance to alkylating agents, bortezomib, and other IMiDs, with overall hematologic response rates ranging from 40% to 60%.74,75 Response rates can be higher when IMiDs are combined with alkylators, but myelosuppression is significant.76-80 Lenalidomide should be used with caution in patients with relevant proteinuria or low eGFR.81 An increase in NT-proBNP has been reported with IMiDs that should be considered when assessing cardiac response.82 The second-generation oral proteasome inhibitor ixazomib has been tested in a phase 1/2 trial in relapsed/refractory patients with AL amyloidosis, showing promising activity, particularly in bortezomib-naive subjects.83 A randomized phase 3 trial comparing ixazomib with physician’s best choice is underway (NCT01659658). An additional option for relapsed/refractory patients is bendamustine. This drug has been tested in a prospective trial and in a retrospective series, granting a hematologic response in 40% to 50% of patients.84,85 Bendamustine can be particularly effective in immunoglobulin M-AL amyloidosis. Novel anti-plasma cell approaches borrowed from multiple myeloma are currently being considered for treating AL amyloidosis. They include the proteasome inhibitor carfilzomib, the anti-plasma cell antibody daratumumab, and the immunostimulatory monoclonal antibody elotuzumab, targeting signaling lymphocytic activation molecule F7. The goal of suppressing the production of amyloidogenic light chains also may be achieved with small interfering RNA targeting the light chain constant region.86 In turn, this can lead to plasma cell death resulting from terminal endoplasmic reticulum stress in clones producing an intact immunoglobulin. The possibility of exploiting the intracellular quality control mechanisms to selectively reduce the secretion of misfolded light chains has been recently investigated, with encouraging results.87 An innovative and very promising approach conceptually derived from the studies on the stabilization of the transthyretin tetramer is aiming at stabilizing the amyloidogenic light chains with small ligands to inhibit their aggregation.88
Targeting the amyloid deposits and interfering with amyloidogenesis and organ damage
Nonchemotherapy approaches to the treatment of AL amyloidosis are now rapidly expanding. We demonstrated that a small molecule, the anthracycline 4′-iodo-4′-deoxy-doxorubicin, inhibited amyloidogenesis in vitro and could improve the clinical status and promote resorption of amyloid deposits in patients with AL amyloidosis.89,90 A compound with a molecular structure that closely resembles that of 4′-iodo-4′-deoxy-doxorubicin, the antibiotic doxycycline, was also shown to disrupt amyloid fibrils in vitro and to reduce the amyloid load in a transgenic mouse model.91 Moreover, we recently showed that doxycycline is capable of counteracting the proteotoxicity of amyloidogenic light chains in the C. elegans model.30 This is relevant to the recent preliminary report that the addition of doxycycline to chemotherapy improved survival of patients with stage II/IIIa cardiac AL amyloidosis in a small, retrospective case-control study in which patients were matched based on the severity of the disease, but not chemotherapy regimen received.92 Doxycycline has the advantage of being a marketed drug that can be “repurposed” for the treatment of amyloidosis, and an international randomized trial comparing chemotherapy plus doxycycline versus chemotherapy alone is being designed.
The use of polyphenols as inhibitors of fibrillogenesis is also being considered with interest. A case of improvement in cardiac symptoms of AL amyloidosis in a patient purposely drinking high amounts of green tea was reported.93 The clinical activity of epigallocatechin gallate was then confirmed in retrospective case series, and clinical trials are underway (NCT01511263, NCT02015312).94
Pepys et al investigated the possibility of promoting amyloid resorption by depleting serum amyloid P component, a common constituent of amyloid deposits, with a palindromic compound, CPHPC, a competitive inhibitor of serum amyloid P component binding to amyloid fibrils.95 The first pilot study of combined CPHPC and anti-serum amyloid P component antibodies in humans has recently been reported, with encouraging results,96 and a trial based on validated organ response criteria is eagerly awaited. Hrncic et al have also explored immunotherapy of systemic amyloidosis. They showed that infusion of an anti–light chain monoclonal antibody having specificity for an amyloid-related epitope caused the resolution of amyloidomas generated in mice by injection of amyloid proteins extracted from the spleens or livers of patients with AL amyloidosis.97 The encouraging results of the pilot study of this antibody in humans have been recently reported.98 A monoclonal antibody (NEOD001) to a cryptic epitope on amyloid fibrils has been reported to target amyloid deposits and accelerate the regression of AL κ amyloidomas in mice. The first phase 1/2 study of NEOD001 in AL amyloidosis showed that cardiac response rate was 50%, and the renal response rate was 43%.99 A randomized, placebo-controlled phase 3 trial is underway (NCT02312206).
Supportive therapy
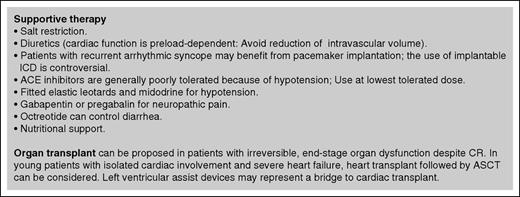
Supportive treatment aimed at maintaining the quality of life and preserving organ function while specific therapy has time to take effect is also critical. Indications are summarized in Figure 3. Supportive treatment is particularly relevant in patients with advanced heart failure, in whom it is vital to sustain cardiac function while effective specific therapy is delivered. Young patients with isolated advanced heart involvement can be considered for heart transplant followed by effective treatment. However, low-dose chemotherapy should be started immediately because survival on the waiting list is dramatically shorter for patients with amyloidosis than for other candidates for heart transplant.100
Supportive therapy in systemic amyloidosis. Supportive treatment is a fundamental part of the management of patients with systemic AL amyloidosis and is aimed at sustaining organ function while specific therapy is delivered, as well as at improving quality of life. Transplantation of the organs involved by amyloidosis may render patients with advanced disease eligible for aggressive specific treatment. The main concerns with organ transplantation are recurrence of amyloidosis in the graft and progression in other organs. However, the availability of effective anticlone treatments and the ever-improving long-term survival of patients with AL amyloidosis allow considering organ transplant in an increasing proportion of patients. Heart transplant followed by ASCT or other effective chemotherapy can be the only effective option for young patients with isolated, severe cardiac involvement. Moreover, organ transplant can be considered in patients who attain complete response, but have irreversible end-stage organ damage. ACE, angiotensin-converting enzyme; ICD, implantable cardioverter defibrillator.
Supportive therapy in systemic amyloidosis. Supportive treatment is a fundamental part of the management of patients with systemic AL amyloidosis and is aimed at sustaining organ function while specific therapy is delivered, as well as at improving quality of life. Transplantation of the organs involved by amyloidosis may render patients with advanced disease eligible for aggressive specific treatment. The main concerns with organ transplantation are recurrence of amyloidosis in the graft and progression in other organs. However, the availability of effective anticlone treatments and the ever-improving long-term survival of patients with AL amyloidosis allow considering organ transplant in an increasing proportion of patients. Heart transplant followed by ASCT or other effective chemotherapy can be the only effective option for young patients with isolated, severe cardiac involvement. Moreover, organ transplant can be considered in patients who attain complete response, but have irreversible end-stage organ damage. ACE, angiotensin-converting enzyme; ICD, implantable cardioverter defibrillator.
Conclusion
The diagnosis and treatment of AL amyloidosis, as well as the efficient conduction of clinical trials, require adequate technology and experience, and patients should be referred to specialized centers and enrolled in clinical trials whenever possible. In the last few years, the availability of reliable diagnostic tools, the establishment of biomarker-based risk assessment, and evaluation of response and novel therapeutic agents have reshaped the approach to patients with AL amyloidosis. We are constantly improving long-term outcomes, several groups are studying the effect of minimal residual disease in AL amyloidosis, and we can hope that some of our patients may eventually be cured. However, treatment of patients with advanced cardiac involvement remains a largely unmet need, and every effort should be made to increase the proportion of patients who are diagnosed at earlier, treatable stages. Although the number of ongoing trials is encouraging, our treatment approach is still largely based on uncontrolled studies. However, the results of the ongoing trials, coupled with a better understanding of the amyloid clone, will help establishing the role of novel agents. Moreover, the clarification of the mechanisms of organ damage will identify potential alternative therapeutic targets. Thus, we can hope to witness an early dawn of effective patient-tailored treatment in AL amyloidosis.
Acknowledgments
This study was supported in part by grant from “Associazione Italiana per la Ricerca sul Cancro–Special Program Molecular Clinical Oncology 5 per mille n. 9965,” from Cassa di Risparmio delle Provincie Lombarde (CARIPLO) “Structure-function relation of amyloid: understanding the molecular bases of protein misfolding diseases to design new treatments n. 2013-0964,” and from CARIPLO “Molecular mechanisms of Ig toxicity in age-related plasma cell dyscrasias n. 2015-0591.”
Authorship
Contribution: G.P. and G.M. designed the review and wrote the manuscript.
Conflict-of-interest disclosure: G.P. has received travel expenses from Celgene. G.M. has received honoraria from Millennium-Takeda and Pfizer, consulting fees from Janssen, speaker fees from Pfizer, and travel expenses from Janssen and Pfizer.
Correspondence: Giovanni Palladini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: giovanni.palladini@unipv.it.