Abstract
Background: Acute myeloid leukemia (AML) patients with internal tandem duplication of FLT3 (FLT3-ITD AML) routinely develop resistance to FLT3 inhibitor monotherapy. Resistance to inhibitors such as quizartinib can develop through mutation of the FLT3 kinase domain, but not all resistant patients have mutations indicating that additional mechanisms of resistance are important. Although quizartinib induces a rapid reduction of circulating leukemia blasts, residual blasts in the marrow persist, suggesting that the microenvironment protects blasts from quizartinib and may serve as a reservoir for development of resistance.
Results: Using a screen of molecules from the microenvironment, we found that FLT3 ligand (FL) and fibroblast growth factor 2 (FGF2) were the two most protective proteins of FLT3-ITD MOLM14 cells when treated with 10 nM quizartinib in vitro. FL protection has been previously described by Mark Levis' group, so we focused on the mechanism of FGF2 protection. FGF2 bound FGFR1, activating the MAPK pathway to mediate resistance. FGF2-mediated resistance could be overcome by concomitant inhibition of the FGFR receptor using the inhibitor PD173074 or by siRNA knock-down of FGFR1. FGFR1 is highly expressed in primary AML cells, and exogenous FGF2 protected primary FLT3-ITD AML cells from quizartinib ex vivo in a dose-dependent fashion (p=0.0007 at 100 ng/ml).
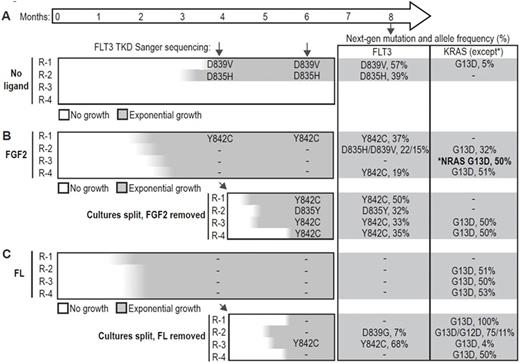
MOLM-14 cells were cultured continuously with quizartinib in media alone, or media supplemented with 10 ng/ml FGF2 or FL to mimic the effects of the microenvironment. MOLM14 cells supplemented with FL or FGF2 resumed growth after 6-8 weeks, significantly faster than cells cultured in media alone, of which only 2/4 developed resistance after 12 weeks. To test the dependence of resistance cultures on exogenous ligand, we removed ligand from the FGF2- and FL-dependent cultures after 4 months and continued quizartinib treatment. After a temporary pause in growth, the cells regained exponential growth within 1 month. We assayed FLT3 resistance mutations by Sanger sequencing during this time, and again at 8 months with a targeted next-generation sequencing panel of commonly mutated genes in AML. Deep sequencing revealed multiple FLT3 mutations along with recurrent mutations of KRAS and NRAS, indicating the importance of the FLT3 and MAPK signaling pathway in resistance (Figure 1). Multiple FLT3 mutations were identified strongly suggesting that FLT3 mutations were not pre-existing but developed during culture with quizartinib (Figure 1). In contrast, the frequency of the KRAS G13D mutation suggested this mutation was present at a low frequency in MOLM14 cells.
FGF2 expression was evaluated by immunohistochemistry in serial bone marrow core biopsies from 10 FLT3-ITD AML patients on the phase II quizartinib trial. Stromal FGF2 expression increased significantly with quizartinib therapy (p<0.01) and remained elevated until patients acquired FLT3 resistance mutations or other intrinsic mechanisms of resistance. Once stromal FGF2 became redundant for survival, expression decreased again. FGF2 protected cells in a paracrine fashion since FGF2 immunofluorescence did not overlap with CD45 (hematopoietic marker) in core biopsies of quizartinib-treated patients.
To test the effect of combined FLT3 and FGFR inhibition in a more complex in vitro model of the microenvironment, MOLM14 cells were co-cultured in transwells over HS-5 (FGF2 high expression) or HS-27 stromal cell lines (FGF2 low). HS-5 stromal cells were highly protective of MOLM14 cells treated with quizartinib alone, but this protection was attenuated by combined FGFR and FLT3 inhibition (p<0.01). In contrast, protection of MOLM14 cells in HS-27 co-culture was minimal, and unaffected by combined FLT3 and FGFR inhibition.
Conclusions: Our data supports a two-step model of resistance to quizartinib with initial resistance mediated by microenvironmental proteins such as FGF2 and/or FL, followed by kinase domain mutation of FLT3 and/or activating mutations of the RAS pathway. Early resistance mediated by FGF2-expressing stroma can be overcome by concomitant inhibition of FLT3 and FGFR suggesting that pre-emptively targeting extrinsic resistance pathways, in combination with newer FLT3 inhibitors that have activity against kinase domain mutations, will improve the durability of response to FLT3 inhibitors.
Agarwal:CTI BioPharma Corp: Research Funding. Kovacsovics:Seattle Genetics: Research Funding. Druker:Agios: Honoraria; Ambit BioSciences: Consultancy; ARIAD: Patents & Royalties, Research Funding; Array: Patents & Royalties; AstraZeneca: Consultancy; Blueprint Medicines: Consultancy, Equity Ownership, Other: travel, accommodations, expenses ; BMS: Research Funding; CTI: Equity Ownership; Curis: Patents & Royalties; Cylene: Consultancy, Equity Ownership; D3 Oncology Solutions: Consultancy; Gilead Sciences: Consultancy, Other: travel, accommodations, expenses ; Lorus: Consultancy, Equity Ownership; MolecularMD: Consultancy, Equity Ownership, Patents & Royalties; Novartis: Research Funding; Oncotide Pharmaceuticals: Research Funding; Pfizer: Patents & Royalties; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.