Abstract
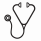
Background
Early response to induction chemotherapy is a significant prognostic factor in the outcome of children with acute lymphoblastic leukemia (ALL). High throughput sequencing (HTS) of rearranged immune receptor (TCR and Ig) genes offers the possibility of a more accurate, sensitive, and standardized approach to determination of early response to therapy.In this study, we investigate the ability of an HTS assay to risk stratify children with B-ALL at the end of induction therapy in comparison with flow cytometry (FC), assess the impact of increased MRD sensitivity on risk group assignment, evaluate the significance of MRD discordance between HTS and FC, and identify a novel subset of patients having an inferior outcome.
Methods
619 paired Pretreatment and End of Induction (Day 29) samples from patients with B-ALL enrolled on Children's Oncology Group (COG) clinical trials AALL0331 (standard-risk, SR) and AALL0232 (high-risk, HR) having minimal residual disease (MRD) at Day 29 of less than 0.1% by flow cytometry were assayed by high throughput sequencing of CDR3 regions of IGH and TCRG. Dominant clonal CDR3 sequences in the pretreatment samples were quantitated in the paired Day 29 samples as residual disease of total nucleated cells without knowledge of the FC results. The relationship of residual disease determined by HTS and FC to 5-year event-free and overall survival (EFS and OS) was evaluated using Kaplan-Meier statistics.
Results
HTS detected a dominant clonal sequence in 93.2% of Pretreatment B-ALL samples, providing an informative cohort of standard-risk (N=282) and high-risk (N=297) patients. Using a threshold of 0.01% on the combined cohort, HTS and FC show identical EFS and OS for MRD positive (77.7% ± 0.04, 91.6% ± 0.03) and negative (92.5% ± 0.02, 96.3% ± 0.01) subsets, see Figure 1. Interestingly, reducing the HTS threshold from 0.01% to 0.0001% results in an improvement in EFS for the HTS MRD positive subset in both standard (80.1% -> 88.2%) and high-risk (75.3% -> 84.8%) patients, likely due to major reductions in the number of patients otherwise scored as MRD negative using the higher threshold of 0.01%(70.9% -> 27.0% SR and 78.5% -> 36.7% HR). This reflects the much more favorable outcome of the large cohort of patients with MRD between 0.0001% and 0.01% compared to those >0.01%. Little improvement in EFS is seen for HTS MRD negative patients with a reduction in MRD threshold. Maximal difference in EFS is achieved at an HTS threshold of 0.01%. Importantly, the subset of SR patients with no detectable residual clonal sequence at any level (19.9% of total) show an excellent EFS (98.1% ± 0.02) and OS (100% ± 0), different from the similar subset of HR patients (30.0% of total) showing less favorable EFS (92.7% ± 0.04) and OS (95.1% ± 0.03). Patients discordant for MRD at a threshold of 0.01%, either HTS+/FC- (N=55) or HTS-/FC+ (N=17), show intermediate EFS compared with concordantly positive or negative patients. Of interest, patients lacking a detectable clonal IgH sequence (N=42) show a significantly inferior EFS (78.5% ± 0.08 vs. 89.3% ± 0.02, p=0.01) but not OS.
Conclusions
HTS is equivalent to FC in its ability to risk stratify patients with childhood B-ALL at End of Induction therapy using a MRD threshold of 0.01%. Reducing the HTS MRD threshold below 0.01% does not improve risk stratification, but does allow identification of a small subset of MRD negative standard-risk patients virtually certain to be cured with current therapy. Patients discordant for MRD between HTS and FC have an outcome intermediate between that seen for concordant patients. Patients lacking a detectable clonal IgH sequence, presumably representing a more primitive form of leukemia, show a significantly inferior outcome.
Equivalence of outcomes by high throughput sequencing and flow cytometry for B-ALL patients at a residual disease threshold of 0.01%.
Equivalence of outcomes by high throughput sequencing and flow cytometry for B-ALL patients at a residual disease threshold of 0.01%.
Wood:Pfizer: Honoraria, Other: Laboratory Services Agreement; Amgen: Honoraria, Other: Laboratory Services Agreement; Seattle Genetics: Honoraria, Other: Laboratory Services Agreement; Juno: Other: Laboratory Services Agreement. Kirsch:Adaptive Biotechnology: Employment. Crossley:Adaptive: Employment, Equity Ownership. Williamson:Adaptive Biotechnology: Employment. Borowitz:HTG Molecular: Consultancy; BD Biosciences: Research Funding; Bristol-Myers Squibb: Research Funding; MedImmune: Research Funding. Loh:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding. Robins:Adaptive Biotechnology: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract