Abstract
Background: Patients (pts) compliant with iron chelation therapy (ICT) experience improved organ function and survival (Delea et al. Transfusion 2007;47:1919-29). Once-daily deferasirox (DFX) dispersible tablets (DT) have a well-defined safety and efficacy profile and, compared with parenteral deferoxamine, provide greater adherence, pt satisfaction, and quality of life (Cappellini et al. Clin Ther 2007;29:909-17). However, barriers exist to pt adherence to DFX DT, including GI tolerability and palatability, leading to development of a film-coated tablet (FCT) that can be taken with or without a light meal. The FCT contains the same active substance as DT but different excipients (lactose and sodium lauryl sulfate removed). Here we present results of the randomized, open-label, Phase II ECLIPSE study that evaluated safety of DFX FCT and DT formulations in pts with transfusion-dependent thalassemia (TDT) or IPSS-R very low-, low- or intermediate-risk MDS.
Methods: ICT-naïve or pre-treatedpts aged ≥10 yrs, requiring ICT at DFX DT ≥30 mg/kg/day (TDT) or ≥20 mg/kg/day (MDS), with serum ferritin (SF) >1000 ng/mL, were enrolled. Exclusion criteria: creatinine clearance below contraindication limit per local label (<60 mL/min or <40 mL/min), serum creatinine >1.5xULN, alanine aminotransferase >5×ULN, urine protein/urine creatinine ratio (UPCR) >0.5 mg/mg, or impaired GI function. ICT-naïve pts received DFX DT 20 mg/kg/day or DFX FCT 14 mg/kg/day. ICT pre-treated pts received a DT or FCT dose equivalent to their pre-washout dose. FCT doses are 30% lower than DT doses due to improved bioavailability, conversion factor 1.43. Dose was adjusted based on SF and investigator's judgment after wk 4 for ICT-naïve pts and after 3 months for ICT pre-treated pts (DT ±5-10 mg/kg/day, max 40 mg/kg/day; FCT ±3.5-7 mg/kg/day, max 28 mg/kg/day); dose adjustments for safety reasons were permitted at any time. Primary endpoint was overall safety, measured by frequency and severity of AEs and changes in laboratory values from baseline (BL) over 24 wks. A secondary endpoint was evaluation of selected GI AEs.
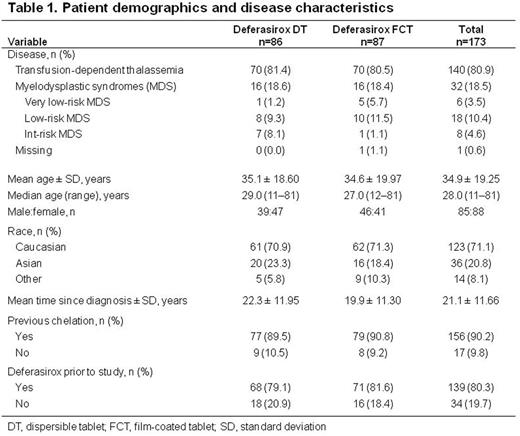
Results: 173 pts were randomized 1:1 to DT or FCT (Table 1). Mean actual daily DFX dose ± SD over 24 wks was 27.5 ± 7.7 mg/kg/day (DT) and 20.8 ± 5.4 mg/kg/day (FCT). More FCT pts were started on a dose higher than the protocol-specified dose than DT pts (26.4% vs 9.3%). Mean/median duration of exposure was 155/168 days with DT and 163/169 days with FCT. 73 (84.9%; DT) and 77 (88.5%; FCT) pts completed 24 wks. Relative consumed tablet count was high, but lower with DT (85.3%) than FCT (92.9%). Absolute median (range) change in SF at end of treatment was -85.5 (-2146 to 8250) ng/mL (from 2485 [915-8250] ng/mL at BL) with DT, and -350 (-4440 to 3572) ng/mL (from 2983 [939-8250] ng/mL at BL) with FCT, corresponding to a median relative change of -4.1% (DT) and -14.0% (FCT) at end of treatment.
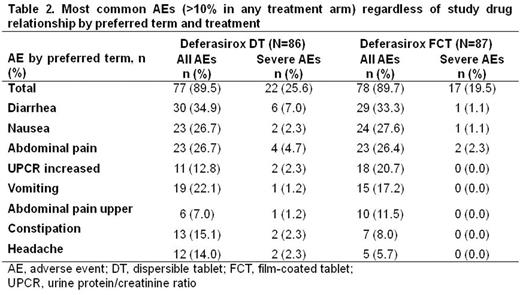
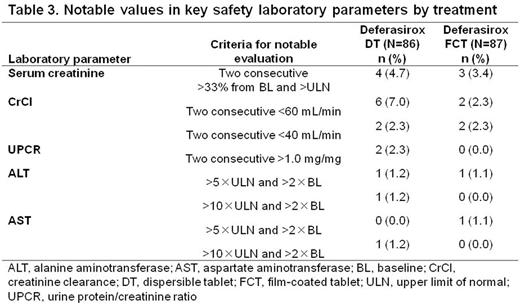
AEs regardless of causality were reported in similar proportions of pts for each formulation (Table 2). Fewer severe AEs were observed in FCT pts. The most common AEs with either formulation were consistent with the known DFX safety profile. Notable laboratory evaluation frequencies were similar with both formulations (Table 3).
61.6% (95% CI, 50.5-71.9%) of DT pts and 58.6% (95% CI, 47.6-69.1%) of FCT pts reported ≥1 GI AEs. Similar proportions of pts had diarrhea, nausea, and abdominal pain. Fewer FCT pts had constipation and vomiting than pts receiving DT. Fewer pts had severe GI AEs with FCT than with DT. In pts receiving DT prior to study entry, fewer FCT pts had GI AEs (53.5%, FCT; 60.3%, DT), particularly diarrhea, vomiting and constipation. Increased UPCR was reported in more FCT pts (12.8%, DT; 20.7%, FCT), which could be attributed to more FCT pts receiving a higher than recommended starting dose. Renal event frequency was similar when patients were started on a correct starting dose (30.9%, DT; 33.3%, FCT). 23 pts discontinued, 10 because of AEs (n=6, DT; n=4, FCT).
Conclusions: These results in pts with TDT or very low-, low- or int-risk MDS demonstrate comparable FCT and DT safety profiles, consistent with the known DFX profile. In patients with prior DT exposure, fewer GI AEs were seen with FCT than DT. Pts receiving FCT had better compliance, continued longer on treatment and experienced greater SF reduction. DFX FCT may improve pt experience with ICT resulting in greater compliance and reduced frequency and severity of iron overload-related complications.
Taher:Novartis: Honoraria, Research Funding; Celgene: Research Funding. Origa:Novartis: Honoraria; Apopharma: Honoraria. Kouraklis:Gilead: Consultancy; Janssen: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Roche: Consultancy; Celegene: Consultancy. Kattamis:Novartis: Honoraria, Research Funding; ApoPharma: Honoraria. Cortoos:Novartis: Employment. Huang:Novartis: Employment. Weill:Novartis: Employment. Herranz:Novartis: Employment. Porter:Novartis: Consultancy, Honoraria, Research Funding; Celegene: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Bluebird Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract