Abstract
Introduction. Haiti is the poorest nation in the Western Hemisphere, and the prevalence of sickle cell disease (SCD) in thatnation is twice that among African-Americans in the United States. Patients with SCD in Haiti have limited access to preventative care and disease management measures due to scarce healthcare resources. Hydroxyurea (HU) is a compelling option for the amelioration of complications of SCD in Haiti due to its relatively low cost, proven safety, and well-documented efficacy. Hydroxyurea programs have been implemented in India and in several African settings, however little data existto demonstrate the acceptability or feasibility of such an effort in Haiti.
Study Design/Objectives. This is an open label, single arm pilot study with the primary objective of examining the acceptability and feasibility of the use of HU to treat children with SCD in an existing pediatric SCD program in Port-au-Prince, Haiti. Acceptability was defined as enrollment of a minimum of two-thirds of patients who are offered participation in the study. Feasibility was defined as two thirds of the enrolled patients being compliant with a defined minimum number of mandated study visits, lab draws, and HU doses. Secondary objectives include documenting the effect of HU on renal, hepatic, and bone marrow function as well as describing the incidence of clinical events in Haitian sickle cell patients taking HU.
Methods. Patients with HbSS disease, age 2-15 years, who met minimum hematologic, renal, and hepatic parameters, were eligible for the study. Patients were approached for inclusion into the study consecutively during three separate enrollment periods from November 2015 through June 2016. The starting dose of Hydroxyurea (capsule and suspension form were available) was 20mg/kg which was increased to a maximum dose of 25mg/kg. Study visits occurred every 4 to 8 weeks at which point laboratory and clinical efficacy parameters, as well as potential adverse effects history were collected and dose modifications occurred. The study period for each patient will last 1 year. Akron Children's Hospital (ACH) IRB and the Haitian National Ethics Board approved the study. Funding for this project is provided through grants from the American Academy of Pediatrics and the ACH Foundation.
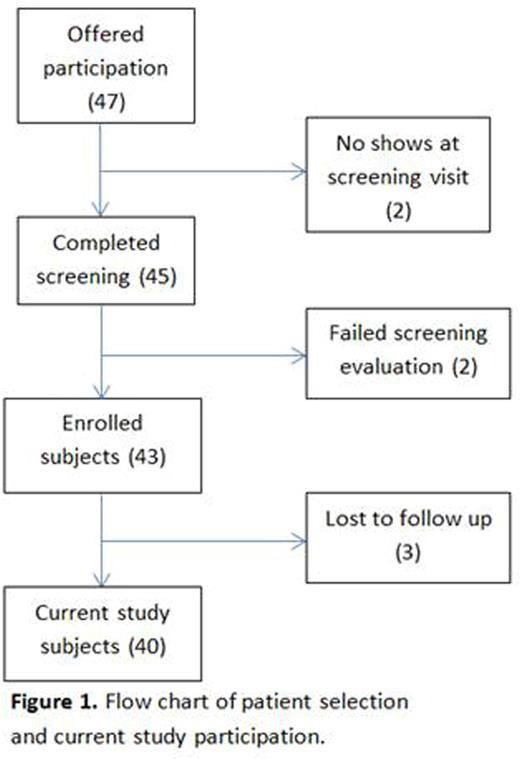
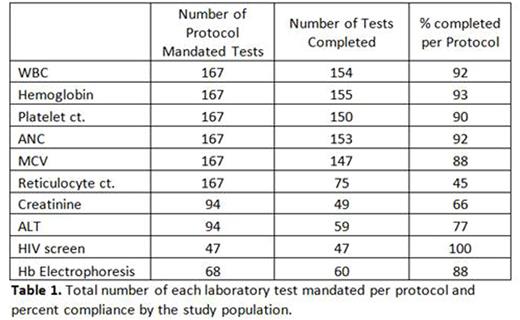
Results. The study is ongoing with the enrollment period being closed as of June 2016. Forty-three patients have been enrolled, with a mean length of participation of 17.6 weeks (range 0-32 weeks).Forty-seven patients were offered participation in the study and 45 signed consent and underwent the screening process, generating an acceptability measure of 95.7%. Two out of the 45 screened patients were excluded based on results from screening labs (1 non-HbSS on confirmatory electrophoresis, 1 severe anemia) resulting in the final enrollment of 43 patients (23M:20F, mean age 9 years). Feasibility is being actively assessed.There have been no serious adverse events and no deaths. Three out of 43 enrolled patients were lost to follow-up and removed from the study due to missing 3 consecutive study visits (see figure 1). Compliance with mandated study visits was high among the enrolled patients with an attendance of 92.9% of the visits. Percent attainment of mandated laboratory tests is shown in table 1. No patients have had HU dose interruptions based on abnormal lab tests. Sixteen study patients have 6 month hematologic laboratory data available at this time: mean Hemoglobin and MCV have increased from 7.1 to 7.9g/dL and 90.6 to 107.1fL, respectively, and mean WBC and platelet count have decreased from 18.0 to 12.4(10^9/mL) and 557 to 413(10^9/mL), respectively.
Conclusion. Results suggest that HU isan acceptable option for treating children with sickle cell disease in Haiti. Our preliminary data show that HU is feasible, safe, and effective in this setting. Challenges exist in ensuring reliable laboratory follow-up and will likely have to be addressed on an individual clinic and laboratory basis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.