Abstract
Introduction: The prevalence of venous thrombosis and pulmonary embolism (VTE) increases with age. Definitive VTE diagnosis or exclusion relies on imaging modalities, which are found to be negative in a high proportion of tested patients. Algorithms based on pretest clinical probability (low and intermediate) in conjunction with negative d-dimer test results have reliably excluded VTE sparing the need for additional imaging studies. Currently, the cutoff for a negative d-dimer is based on reference ranges regardless of age of the donor population. Since baseline d-dimer increases with aging, an age-adjusted d-dimer (AADD) cutoff for patients older than 50 years (age*10 ng/mL FEU) has been shown to improve specificity of diagnosis while still maintaining a high sensitivity (>97%) of detecting VTE/PE (Righini, et al. JAMA, 2014). Due to FDA restrictions, clinical laboratories cannot implement and report such AADD cutoffs. Major hurdles include: AADD cutoffs are assay-dependent, and there is a lack of feasible strategy for laboratories to verify the AADD cutoffs. We hypothesize that an adequate study of age-distribution of baseline d-dimer levels of healthy donors can assist the verification of AADD cutoffs and ultimately ensure patient safety in the VTE patient population.
Objective: Establish an age dependent reference range for patients 50 years or older with the HemosIL HS 500 D-dimer reagent on the ACL TOP 700 (Instrumentation Laboratory, Bedford MA, USA); and compare this range with the manufacturer provided and literature recommended AADD cutoffs.
Methods: D-dimer assay was performed on plasma samples from normal adult donors per the manufacturers' instructions. D-Dimer age, gender distribution and association were analyzed using non-parametric Wilcoxon rank sum, Kolmogorov-Smirnov tests, and quantile regression methods to estimate the 95th percentile to establish a reference range.
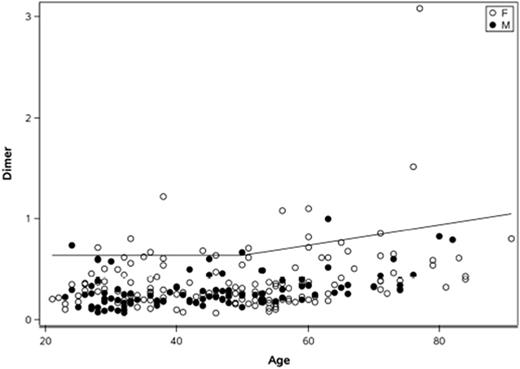
Results: We studied 241 healthy donors, ranging from 21-91 years of age. Statistically significant differences between males and females (100 [42%] male) were detected (p-value <0.001; p-value =0.0009). Using CLSI guidelines, these statistical differences (median d-dimer for men = 200, while median d-dimer for women = 300) are not considered clinically significant, since only 6% of women would be outside an overall reference range (CLSI guideline states to partition if over 8% for either group would be outside the overall reference range). Using the quantile regression method, a significant association between d-dimer and age was found at the median (P<0.0001). We then established a reference range (95th percentile) for donors younger than age 50 at 635 ng/mL FEU for both men and women and AADD reference range for patients 50 and older (635 + (age - 50)*10 ng/mL) (Figure 1). This reference range was verified with our sample set, with only 6.2% of donors higher than the established reference limit. If we used the non-AADD cutoff provided by the manufacturer for donors younger than 50 (500 ng/mL FEU) and the literature recommended AADD cutoffs for older donors (age*10 ng/mL FEU), 13.3% of our normal donors were above the limits, resulting in not validating this literature reference range per CLSI guidelines in EP28-A3c. These data imply that if both manufacturer and literature recommended AADD cutoffs were adopted, HemosIL HS 500 D-dimer assay should have sufficient sensitivity and negative predictive value, although with slightly lower specificity than those reported by Righini, et al. for detecting VTE/PE.
Conclusion: Our study verifies the association between d-dimer level and age and for the first time, to our knowledge, determined AADD normal range for the HemosIL HS 500 D-dimer assay. The manufacturer and literature recommended AADD cutoffs should provide sufficient sensitivity and negative predictive value for VTE/PE exclusion.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.