Abstract
Down-regulation of CD20, a molecular target for monoclonal antibodies, is a clinical problem leading to decreased efficacy of anti-CD20-based regimens. Moreover, a substantial number of patients do not fully benefit from anti-CD20 immunotherapy due to the low CD20 expression. It has been shown that histone deacetylase inhibitors (HDACi), in a transcription-dependent mechanism, regulate CD20 surface level. However, non-specific HDAC pan-inhibitors have considerable number of side effects, therefore a new isoform-specific inhibitors are being developed. Recently, HDAC6-selective inhibitors have emerged in non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) potential therapies. Preclinical data show therapeutic potential of HDAC6 inhibitors in combination approach. However, their effect on the efficacy of anti-CD20 immunotherapy has not been studied so far. Therefore, this study aimed to investigate the influence of HDAC6 inhibition on the regulation of CD20 expression.
This study was performed in in vitro settings using a panel of established B-cell-derived tumors (Raji, Daudi, Ly-1, Karpas) as well as EBV-transformed cells and CLL primary samples. Commercial HDAC6 inhibitors- tubacin, tubastatin and clinically available ricolinostat (ACY1215) were used. In order to silence HDAC6 expression we used shRNA. The expression levels of surface antigens were assessed by flow cytometry. Total protein level was assessed by Western blotting. The influence of HDAC6 inhibition on transcription of CD20 mRNA was analyzed in qRT-PCR. In order to investigate the stability of CD20 protein a cycloheximide chase assay has been employed. The synthesis of protein de novo was investigated using Click-chemistry. To investigate the impact of HDAC6 inhibition on translation of CD20 mRNA polysomes were isolated.
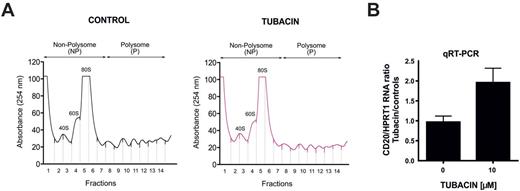
In this study using pharmacological inhibitors and genetic modification we have shown that HDAC6 inhibition significantly increases CD20 level in a panel of B-cell derived human tumor cell lines as well as in primary CLL samples. The observed upregulation of CD20 correlates with sensitization to type I and type II anti-CD20 antibodies. We observed that HDAC6 inhibition increases total CD20 protein level without affecting its mRNA level or promoter activity. As HDAC6 has been shown to regulate protein trafficking and degradation, we performed experiments assessing the influence of HDAC6 inhibition on CD20 endocytosis. We concluded that HDAC6 inhibition does not alter the internalization rate of CD20. In cycloheximide chase assay we showed that CD20 is a rather stable protein and therefore hypothesized that HDAC6 inhibition may be a consequence of increased translation of CD20 mRNA. Using Click-it metabolic labelling we concluded that HDAC6 inhibition significantly increased the production of CD20 de novo. To substantiate our findings on increased translation of CD20 after HDAC6 inhibition, we performed polysomal profiling followed by qRT-PCR on polysomal extracts from Raji cells. We observed no considerable changes in polysomal profiling curves between control and tubacin- pretreated cells further confirming that tubacin did not alter the overall level of protein translation. Importantly, our findings showed a significant increase in CD20 mRNA in polysomal fraction in tubacin-treated group when compared with control cells.
Altogether, our results clearly indicate that HDAC6 is involve in posttranscriptional regulation of CD20 and HDAC6 inhibition increases the translational efficiency of CD20 mRNA. Our results strongly support that HDAC6 inhibition can be a therapeutic strategy successfully enhancing the efficacy of anti-CD20 mAbs. Our pre-clinical investigation might be relevant in terms of designing clinical combination studies and support further use of HDAC6 inhibitors in combination with anti-CD20 mAbs.
Polysomal profiling.
Cytosolic lysates from control and tubacin- pretreated Raji cells were prepared in lysis buffer followed by two centrifugations. Cytosolic proteins were layered onto linear sucrose gradient and sedimented by a 2h centrifugation. The gradients were collected in 15 fractions and absorbance profiles were generated at 254 nm (A). Polysomal fractions were pooled for further RNA extraction and quantitative PCR (B).
Polysomal profiling.
Cytosolic lysates from control and tubacin- pretreated Raji cells were prepared in lysis buffer followed by two centrifugations. Cytosolic proteins were layered onto linear sucrose gradient and sedimented by a 2h centrifugation. The gradients were collected in 15 fractions and absorbance profiles were generated at 254 nm (A). Polysomal fractions were pooled for further RNA extraction and quantitative PCR (B).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.