Abstract
Background: Acute myeloid leukemia (AML) is an aggressive hematologic cancer characterized by clonal proliferation of hematopoietic stem and progenitor cells that exhibit impaired differentiation. Event free survival for patients with AML remains poor despite intensive myelosuppressive therapies and improvements in supportive care measures. This underscores the need for novel, biologically based therapies. Somatic mutations that deregulate epigenetic programs (e.g. DNMT3A, TET2, IDH1/2, EZH2, ASXL1) and signal transduction pathways (e.g., FLT3, NRAS, KRAS) frequently coexist in AML. While the former class of mutations is hypothesized to promote a chromatin state that is permissive for AML development and essential for leukemia maintenance, experimental data also suggest that signaling mutations play a central role in driving leukemic growth in vivo. Thus, simultaneously targeting the abnormal epigenetic landscape and aberrant signaling pathways in AML is a rational new therapeutic approach. Recent publications identified the bromodomain and extraterminal (BET) proteins, an important class of epigenetic reader proteins, as particularly promising therapeutic targets in AML. While these studies support the therapeutic potential of BET inhibition in AML, they have limitations. These include their dependence on exogenous overexpression of oncogenes, failure to inform potential combination therapeutic strategies, and a reliance on monoclonal in vitro systems that do not recapitulate the inherent genetic heterogeneity of human cancers.
Methods: We previously generated a heterogeneous collection of murine AMLs by infecting Nras, Kras, and Nf1 mutant mice with the MOL4070 retrovirus, which exhibit distinct retroviral integrations that are maintained upon transplantation into sublethally irradiated recipient mice. We first established 15 mg/kg/day as the maximally tolerated dose of PLX51107, a selective and potent BET inhibitor, in sublethally irradiated mice in a C57Bl/6 x 129sv/J strain background. We performed pharmacokinetic analysis, which demonstrated excellent drug exposure at doses of 10 and 15 mg/kg/day. We next treated cohorts of recipient mice with PLX51107 (10 mg/kg/day) and in combination with the MEK inhibitor PD0325901 (PD901; 1.5 mg/day). Mice that appeared ill were euthanized and underwent full pathological examination. Despite continuous drug treatment, all recipient mice eventually succumbed to progressive AML.
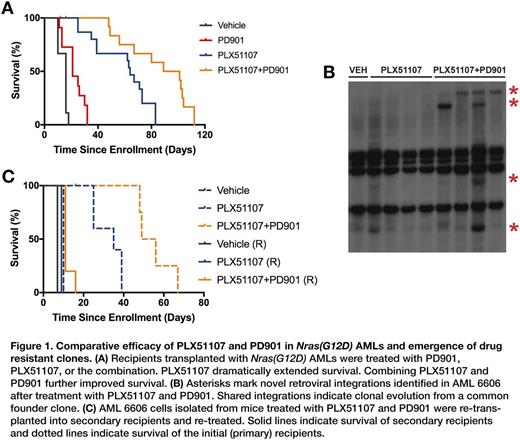
Results: We enrolled eight AMLs, including four with a Nras(G12D) mutation, two with a Kras(G12D) mutation, and two with Nf1 inactivation. Recipient mice received 450 cGy of sublethal irradiation followed by 2x10E6 leukemia cells via tail vein injection. Recipient mice were randomized to receive vehicle (n = 4 for each AML), PLX51107 (n = 5), or PLX51107+PD901 (n = 5). PLX51107 markedly extended the survival of recipients transplanted with Nras(G12D) AMLs 6695, 6606, and 6613 that was further enhanced by PD901 (Fig. 1A). Whereas, PD901 resulted in a 1.5-fold increase in survival over vehicle-treated mice, PLX51107 alone resulted in a 4-fold increase in survival and PLX51107+PD901 in a nearly 6-fold increase in survival in this cohort of Nras(G12D) AMLs. Surprisingly, the response to PLX51107 was blunted in Kras(G12D) and Nf1 inactivated AMLs compared to Nras(G12D) AMLs. The observation of novel MOL4070 integration sites in relapsed AMLs provided definitive evidence of clonal evolution (Fig. 1B). Importantly, we went on to show that drug-treated clones emerging at relapse demonstrate intrinsic drug resistance by re-transplanting these leukemias into secondary recipients and re-treating them in vivo (Fig. 1C).
Conclusion: PLX51107 shows impressive efficacy in a panel of primary AMLs treated in vivo that is further enhanced by PD901. The differential response between Nras(G12D) and Kras(G12D)/Nf(-/-) AMLs leads to the intriguing and unexpected hypothesis that the type of hyperactive Ras signaling mutation may influence the response to BET inhibition in AML. We are interrogating relapsed AMLs to identify and functionally validate candidate mechanisms underlying drug response and resistance through the use of established strategies to directly compare untreated and relapsed leukemias. Ongoing studies include assessing retroviral integrations and performing Western blotting, whole exome sequencing, RNA-seq, and ChIP-seq.
Powell:Plexxikon: Employment. Bollag:Plexxikon Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.