Abstract
Background Early occurrence of severe (≤ 10 g/dl) anemia has been reported in about 5% of newly diagnosed patients with Chronic Myeloid Leukemia (CML) treated with imatinib and is generally transient: in patients responsive to imatinib, however, severe late anemia seems to occur in a higher rate and seems to be more common in elderly patients.
Methods To highlight this issue, we revised retrospectively 81 CML patients aged > 60 years treated at our Institution with frontline imatinib for at least 24 months who achieved a durable complete cytogenetic response (CCyR). The main clinical features of the patients at diagnosis were as follows: male/female 42/39 (51.9%/48.1%), median age 70.5 years [interquartile range (IQR) 64.5 - 76.5], median Hb 12.8 g/dl (IQR 11.2 - 13.7), median WBC 42.5 x 109/l (IQR 25.4 - 78.9), median PLTS 448 x 109/l (IQR 282 - 717). According to Sokal risk classification, 12 patients (14.8%) were low risk, 58 (71.6%) intermediate risk, 11 (13.6%) high risk.
Severe late chronic anemia was defined as the presence of persistent (> 6 months) and otherwise unexplained (creatinine level < 2 mg/dl, normal iron balance, bilirubin level < 2 mg/dl, folate and Vitamin B12 in the normal range) Hb levels ≤ 10 g/dl which occurred > 6 months from imatinib start.
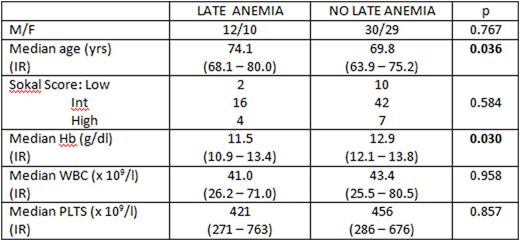
Results On the whole, a condition of late chronic anemia occurred in 22 out 81 patients (27.2%) at different intervals from imatinib start: the incidence was 13.5% (11/81 patients) at the 12th month, 14.8% (12/81 patients) at the 24th month, 12.3% (9/73 patients) at the 36th month, 7.0% (4/57 patients) at the 48th month and 5.9% (3/51 patients) at the 60th month. Seven out 22 patients (31.8%) needed packed red cell transfusions during the follow-up. Clinical features at diagnosis of patients who had severe late chronic anemia compared to the remaining 59 patients without anemia are shown in the Table:
Among the 22 patients with severe late chronic anemia, 6 were treated with subcutaneous recombinant alpha-erythropoietin (EPO) at the standard dosage of 40,000 UI weekly, after a median time from imatinib start of 44.7 months (absolute range 26.8 - 73.8): in all 6 patients, baseline endogenous EPO levels were < 100 mUI/ml. All 6 patients achieved erythroid response, which was complete (achievement of stable Hb levels > 12 g/dl) in 4 patients and partial (stable increment > 1.5 g/dl of Hb levels with Hb levels < 12 g/dl) in 2 patients: one patient had a relapse of anemia after 24.1 months from EPO start and stopped EPO treatment, the remaining 5 patients are still in response and in treatment with EPO. No EPO-related toxicity was observed.
Cumulative 4-year Event-Free Survival (EFS) for patients with severe late chronic anemia was 69.7% (CI95% 49.3 - 90.1) compared to 86.2% (CI95% 77.4 - 95.0) for patients without anemia (p=0.075): cumulative 4-year Overall Survival (OS) for patients with severe late chronic anemia was 89.6% (CI95% 75.9 - 100) compared to 94.8% (CI95% 89.1 - 100) for patients without anemia (p=0.204).
Conclusions The occurrence of a late severe chronic anemia during long-lasting treatment with imatinib has been observed in > 25% of our responsive elderly patients, with a trend for a worse EFS: its occurrence seems more common in very elderly patients with lower Hb levels at diagnosis. Results with EPO are encouraging, but larger studies are warranted to define the role of such an approach in treating this common late complication of prolonged imatinib therapy.
Breccia:Celgene: Honoraria; Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Ariad: Honoraria; Pfizer: Honoraria. Latagliata:Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Janssen: Consultancy, Honoraria; Shire: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.