Abstract
BACKGROUND
Early molecular response (EMR) of less than 10%IS of BCR-ABL transcript level at 3 months has become widely accepted as the earliest milestone in CML treatment. Close monitoring and switch of TKI therapy is recommended if the patient continues to fail to achieve less than 10%IS at 6 months. Thus EMR has guided our treatment intervention in the high risk group of patient before they progress to advanced stage CML. After multiple TKI therapy failure, the only available curative treatment is allogeneic hematopoietic cell transplantation (HCT). Around 20-30% of patients still progress or relapse after HCT. Accordingly, there is obligatory requirement for early molecular monitoring to guide pre-emptive treatment to reduce the risk of imminent relapse. The current study reviewed and analyzed transplant outcomes in 223 CML patients.
METHODS
A total of 223 patients were reviewed who received HCT across 6 transplant centers in Canada between 2002 and 2014. According to their response to TKI therapy and disease state prior to HCT, patients were divided into 4 groups: TKI resistance (n=132), TKI intolerance (n=29), TKI naïve/response chronic phase (CP) (n=27) or advance disease stage but TKI responsive (n=35). Using the binary recursive partitioning method with BCR-ABL transcript levels taken within 3 months post-HCT, patients were further divided into 3 groups: 1) those failing to achieve less than 1.3 log reduction (Group1.3), 2) the group achieving between 1.3 and 4.4 log reduction (Group1.3-4.5), and 3) the patients who achieved 4.5 log reduction or deeper response (Group4.5). According to the 4 groups stratified by TKI response/disease risk or to the 3 groups based on BCR-ABL transcript level within 3 months, transplant outcomes were analyzed with respect to overall survival (OS), non-relapse mortality (NRM), relapse and incidence of complete hematologic response (CHR) loss. Post-HCT TKI therapy for relapsed CML was also reviewed and analyzed for response/long-term outcomes.
RESULTS
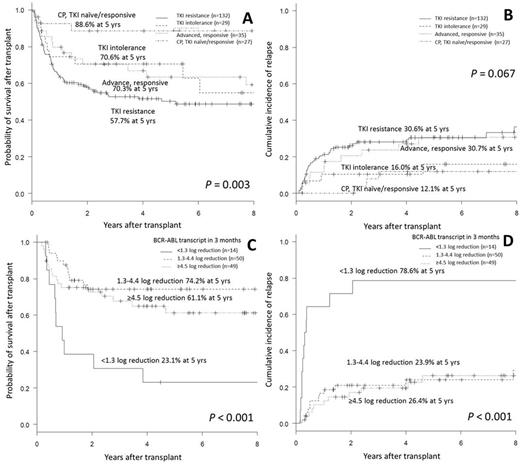
The OS was the highest in TKI naïve/responsive CP group (88.6% at 5 years), followed by TKI intolerance (70.6%), advanced disease group responsive to TKI therapy (63.4%) and the lowest in TKI resistance group (50.3%; p=0.003 among 4 groups). The incidence of relapse was high in TKI resistance group (30.6% at 5 years) and in advanced disease group (30.6%) compared to TKI intolerance group (16.0%) or TKI naïve/responsive CP group (12.1%; p=0.067).
According to BCR-ABL transcript levels 3 months post-HCT, Group1.3 showed the lowest rate of molecular response (MR) 4.5 (7.1% at 2 years) compared to 49.9% in Group1.3-4.5 vs 72.7% in Group 4.5 (p<0.001). Group1.3 also showed the highest relapse rate of 78.6% at 5 years, compared to 23.9% in Group 1.3-4.5 and 26.4% in Group 4.5 (p<0.001).
In multivariate analysis for OS, Group1.3 increased the risk of death by 2.3 fold in addition to advanced disease at diagnosis (HR 2.5), acute GVHD (HR 2.3) and chronic GVHD (HR 0.28). For relapse, Group1.3 increased the risk of relapse by 6.6 fold, while chronic GVHD decreased it by 57% (HR 0.43). However, none of TKI failure, disease stage prior to HCT, additional cytogenetic abnormalities in Ph+ clone, clonal evolution in Ph neg clone, or ABL1-kinase domain mutation was found to be prognostic.
Of 223, 61 patients experienced relapse in 10 months post-HCT, among which 47 patients were treated with TKI therapy. Of 45 evaluated, 26 patients (57.8%) achieved molecular response. Response was lower in the group with CHR loss as 34.6% than those with MR2 loss (100%) or MR3 loss (88.2%; p<0.001). For OS after post-HCT TKI therapy (OSTKI), OSTKI was the highest in the group with MR3 loss (81.9%), followed by those with MR2 loss (66.7%), then lowest with CHR loss (27.0%; p<0.001). Multivariate analysis confirmed that relapse type is an independent risk factor for response (p=0.002) and for OSTKI (p=0.004). The group with hematologic relapse as CHR loss had 8.9 times higher risk of death compared to those with loss of MR2/MR3.
CONCLUSION
The present study confirms that the EMR within 3 months post-HCT is associated with transplant outcomes: failure to achieve 1.3 log reduction of BCR-ABL transcripts in 3 months post-HCT for CML, significantly increases the risk of relapse. Thus pre-emptive post-HCT TKI therapy is warranted in failing patients to reduce imminent risk of relapse after allogeneic HCT in CML patients.
Savoie:Jazz: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Speakers Bureau; Lundbeck: Consultancy. Bence-Bruckler:Lundbeck: Membership on an entity's Board of Directors or advisory committees. Lalancette:Celgene: Honoraria; BMS: Honoraria. Hillis:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.