Abstract
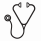
Systemic mastocytosis (SM) is a myeloid neoplasm defined by abnormal growth and pathologic accumulation of neoplastic mast cells (MC) in various internal organs. The indolent variant of SM (ISM) is associated with an almost normal life expectancy. By contrast, the prognosis in advanced SM, including SM with an associated hematologic neoplasm (AHN), aggressive SM (ASM), and MC leukemia (MCL) is poor with short survival times. Most patients with SM express the D816V-mutated variant of KIT, which confers resistance against several tyrosine kinase inhibitors (TKI), including imatinib. Midostaurin is a TKI that is effective against KIT D816V. However, despite encouraging clinical efficacy, this drug cannot produce continuous complete remission in all patients. One problem in advanced SM is that the AHN component of the disease, especially when progressing into acute myeloid leukemia (AML) is often drug-resistant. The aims of this study were to evaluate the effects of the multi-kinase inhibitor DCC-2618 on proliferation and survival of primary neoplastic mast cells, various mast cell lines and other malignant and non-malignant cell types that may play a role in advanced SM. As assessed by 3H-thymidine-uptake, DCC-2618 was found to inhibit the proliferation of all human MC lines tested, with lower IC50 values measured in HMC-1.1 cells lacking KIT D816V (11.2±4.3 nM) and ROSAKIT WT cells (61±11 nM) than in KIT D816V+ HMC-1.2 cells (147±68 nM) and ROSAKIT D816V cells (133±43 nM). DCC-2618 also produced growth inhibition in the multi-resistant MCL lines MCPV-1.1 (164±72 nM), MCPV-1.2 (256±167 nM), MCPV-1.3 (124±46 nM), and MCPV-1.4 (235±114 nM). In addition, DCC-2618 was found to inhibit the proliferation of primary neoplastic bone marrow MC obtained from patients with SM including MCL (Figure). We also found that DCC-2618 induces apoptosis in HMC-1 cells and ROSA cells, and to a lesser degree in MCPV-1 cells as determined by light microscopy and AnnexinV/PI staining. Moreover, DCC-2618 was found to block phosphorylation of KIT in all MC lines tested. In a next step, we explored the effects of DCC-2618 on growth of other leukemia cell lines as well on vascular endothelial cells. In these experiments, we were able to show that DCC-2618 inhibits the proliferation of the FIP1L1-PDGFRA+ eosinophilic leukemia cell line EOL-1 (IC50 2±0.6 nM) and the FLT3 ITD-mutated AML cell lines MV4-11 (IC50 130±18 nM) and MOLM-13 (IC50 110±26 nM). DCC-2618 also induced apoptosis in EOL-1, MV-411, and MOLM-13 cells. Moreover, DCC-2618 was found to inhibit the growth of cultured human vascular endothelial cells, suggesting that the drug may also counteract SM-related neo-angiogenesis in SM. DCC-2618 did not inhibit the proliferation of the immature AML cell line KG1 and the monoblastic cell line U937, but was found to block proliferation in primary leukemic monocytes in patients with monoblastic AML or chronic myelomonocytic leukemia (CMML) which may have clinical implications as CMML and AML are the most prevalent types of AHN in advanced SM. Finally, we were able to show that the major DCC-2618-metabolite, DP-5439, is equally effective in producing growth inhibition in all cell lines tested as well as in primary neoplastic MC compared to DCC-2618 (Figure). In summary, our data show that DCC-2618 is a new potent multi-targeted TKI that counteracts growth of neoplastic MC as well as growth and survival of leukemic monocytes, AML blasts, eosinophils, and endothelial cells in vitro. Whether DCC-2618 is also able to inhibit the growth of neoplastic MC and other leukemic (AHN) cells in vivo in patients with advanced SM remains to be determined in clinical trials. Indeed, a first Phase I clinical trial examining the effects of DCC-2618 in SM has recently been initiated.
Valent:Novartis: Honoraria, Research Funding; Amgen: Honoraria; Celgene: Honoraria, Research Funding; Ariad: Honoraria, Research Funding; Deciphera Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract