Abstract
Background: Induction chemotherapy followed by autologous stem cell transplantation (ASCT) is considered a standard of care for appropriate patients with newly diagnosed multiple myeloma (MM), but with the rapid expansion of available treatments, the role of ASCT has been questioned. Numerous studies have shown an improvement in complete response (CR) and progression free survival (PFS) with ASCT but our study is a cost comparison between novel agents and ASCT. We aimed to compare the cost effectiveness of ASCT versus non-transplant regimens in relation to PFS.
Methods: We queried the Healthcare Cost and Utilization Project's Nationwide Inpatient Sample (NIS) between 2008 and 2013 using the ICD-9 code 203.00 for MM and V42.81 for ASCT in the primary and secondary diagnosis fields. The analysis included patients who were 18 years or older. We monitored the trends in ASCT with regards to cost of a transplant admission and length of stay (LOS). Cost of hospitalization was adjusted for inflation in reference to the year 2011 and cost to charge ratio. Literature regarding common treatment regimens, response rates, duration of treatment, and PFS was reviewed. The assumption was made that transplant eligible patients that did not undergo transplant would be treated as though they were transplant ineligible. Gay et al 2015 showed a 43.4-month (mo) median PFS with 4 cycles of Rd followed by ASCT and lenalidomide maintenance until progression or toxicity. The cost of novel regimens was estimated using the one month commercial cost described by Roy et al and wholesale acquisition costs for new agents not described by the study which were adjusted using adjunct cost described by the study. A standard weight of 70 kg was used for agents requiring dose calculations. The cost of novel agents for an equivalent duration of 43.4 mo was estimated.
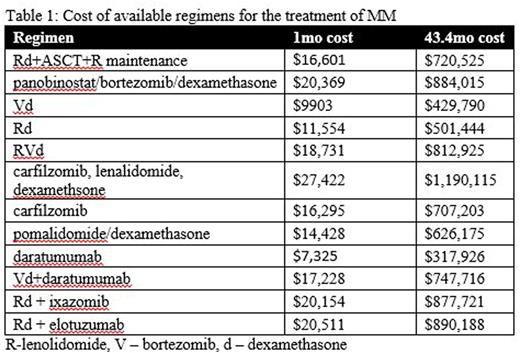
Results: A total of 44778 (weighted n=9039) hospitalizations for MM (Male 55.9%, Caucasian 57.9%, peak incidence 65-79 yrs) occurred from 2008-2013. The average length of stay during ASCT admission was 11.4 +/- 0.4 days with a cost of $109,856 +/-5749.83. There has been a 16.89% decrease in LOS and 1.99% increase in the cost of ASCT (p<0.05). Gay et al showed a 43.4 mo PFS with 4 cycles Rd ($46,216) followed by ASCT ($109,856) and lenalidomide maintenance (~35.4 mo $564,453 adjusted for cost of office visits and lab work) for a total of $720,525
Conclusion:
Rd + ASCT + R maintenance is efficacious for the treatment of newly diagnosed MM. The cost associated with the induction and transplant represents only 22% of the total cost, whereas lenalidomide maintenance makes up 78%. Considering secondary risks and the cost involved with lenolidomide maintenance, further investigation is warranted regarding the optimal duration of maintenance therapy and resultant progression free survival. It also raises the need for alternative options for maintenance therapy. Bortezomib and ixazomib are both more cost effective options and studies are in progress to assess their efficacy in PFS in the maintenance setting. Studies using novel agents for induction prior to ASCT are ongoing and have yet to reach an optimal duration of therapy. Table 1 shows that most of the novel agents used in combination regimens are more expensive that ASCT. Although most patients would not remain on one of these regimens for 43.4 mo, they would presumably progress through multiple lines of therapy during the 43.4 mo of PFS that ASCT provides. In an era of cost consciousness, ASCT should continue to be an integral component of MM treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.