Abstract
Purpose/Background: Oral anticoagulation is recommended for stroke prevention in patients with non-valvular atrial fibrillation (NVAF) and stroke risk factors, but discontinuation rates are high among those treated with vitamin K antagonists (VKA). After the first year of treatment, about half of patients permanently stop taking VKA therapy. We examined persistence to therapy with dabigatran etexilate (DE) in patients enrolled in the global, prospective GLORIA-AF Registry Program.
Methods: GLORIA-AF collects data in three phases from routine clinical practice in 44 countries worldwide. Enrollment in Phase II was initiated following approval of DE, the first non-VKA oral anticoagulant (NOAC) available. During this phase, all patients with newly diagnosed NVAF at risk for stroke starting DE are followed for 2 years.
This analysis is based on a pre-specified interim analysis once follow-up of the first 3000 DE patients was completed. Patients were recruited between November 2011 and December 2013 at nearly 1,000 sites worldwide, by cardiologists, neurologists and general practitioners. To reduce selection bias, patients were recruited consecutively, irrespective of antithrombotic therapy. Persistence was defined as time from initiation to discontinuation of therapy for >30 days or substitution of initial treatment by another oral anticoagulant. Persistence rates were analyzed on the basis of a time-to-event analysis using the Kaplan Meier method.
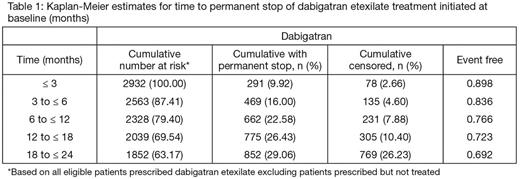
Results: Among eligible patients, 2,937 were prescribed DE; 823 (27.4%) in North America, 1,503 (50.1%) in Europe, 194 (6.5%) in Latin America, 54 (1.8%) in Africa/Middle East and 363 (12.1%) in Asia. Overall, 55.3% were male, the median age was 71.0 (range 23-98) years; 36.7% were ≥75 years old. The CHA2DS2VASc score was ≥2 in 88.2%, 78.9% had hypertension, 22.7% diabetes mellitus, 10.1% prior stoke and 24.9% heart failure. All but 5 eligible patients took at least one dose of DE. The probability of remaining on DE treatment was 76.6% at 1 year and 69.2% at 2 years (based on Kaplan-Meier method). At the 2 years visit, half of the permanently discontinued patients (418 out of 828) had switched to another oral anticoagulant. Characteristics of patients discontinuing vs. sustaining therapy and relationships to stroke risk and geographical region will be presented.
Conclusions: In this global, prospective, cohort of patients newly diagnosed with NVAF and treated with DE, persistence on therapy was high through 2 years of treatment, with an estimated probability of remaining on treatment of about 77% after 1 year and 70% after 2 years. The detailed results will provide a global perspective on the factors that influence treatment persistence in patients prescribed a NOAC for stroke prophylaxis.
Teutsch:Boehringer Ingelheim: Employment. Huisman:Boehringer Ingelheim Pharma GmbH & Co.KG: Other: Grant support; GlaxoSmithKline: Other: Grant support; Bayer HealthCare: Other: Grant support; Pfizer: Other: Grant support; Actelion: Other: Grant support. Lip:Bayer, BMS/Pfizer, Boehringer Ingelheim and Sanofi Aventis: Speakers Bureau; Bayer, Astellas, Merck, Sanofi, Bristol-Myers Squibb (BMS)/Pfizer, Daiichi-Sankyo, Biotronik, Portola and Boehringer Ingelheim: Consultancy. Diener:AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Novartis, Sanofi Aventis, Syngis and Talecris: Research Funding; Abbott, Allergan, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, CoAxia, Corimmun, Covidien, Daiichi-Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, Medtronic, MindFrame, MSD, Neurobiological Technologies: Honoraria; The Department of Neurology at the University Duisburg-Essen received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, National Institutes of Health, Bertelsmann Foundation and Heinz: Research Funding. Dubner:steering committee member for Boehringer Ingelheim: Consultancy; St Jude Medical: Research Funding. Changsheng:steering committee member for Boehringer Ingelheim: Consultancy. Rothman:RTI Health Solutions: Employment. Zint:Boehringer Ingelheim: Employment. Elsaesser:Boehringer Ingelheim: Employment. Paquette:Boehringer Ingelheim: Employment. Bartels:Boehringer Ingelheim: Employment. Halperin:Bayer HealthCare: Consultancy; Boehringer Ingelheim: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.