Abstract
[Background]
Pediatric acute myeloid leukemia (AML) remains to be a disease with a poor prognosis, with a long-term survival rate of approximately 60%. We have recently reported that high PRDM16 expression (high-PRDM16) was common in t(5;11)(q35;p15.5)/NUP98-NSD1-positive patients, and that high-PRDM16 was found to be a novel poor prognostic factor in this disease. However, this finding was not observed in FAB-M7. The presence of inv(16)(p13.3q24.3)/CBFA2T3-GLIS2 was postulated to be a reason. This fusion was reported as the most frequent fusion gene associated with a poor prognosis in FAB-M7, and we found that all CBFA2T3-GLIS2-positive patients had a low PRDM16 expression (low-PRDM16). Furthermore, some studies reported that bone morphogenetic protein 2 (BMP2) was frequently and highly expressed in CBFA2T3-GLIS2-positive patients, indicating the importance of BMP2 in pathogenesis in a subset of AML. In this study, we analyzed the expression status of BMP2 and PRDM16 and their relationship to prognosis in pediatric AML.
[Patients and Methods]
A total of 369 AML patient samples, who were enrolled in the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG) AML-05 trial, were analyzed. Expression level of BMP2 and PRDM16 is analyzed by real-time quantitative PCR (Taqman Gene Expression Assay), using ABL1 as a control gene. The cutoff value for high-BMP2 was defined as the lowest expression level among 11 CBFA2T3-GLIS2-positive patients, and that for high-PRDM16 was defined as the lowest expression level among 10 NUP98-NSD1-positive patients. We also screened fusion genes and gene mutations of CBFA2T3-GLIS2, NUP98-JARID1A, RBM15-MKL1, MLL rearrangements, NUP98-NSD1, FUS-ERG, DEK-NUP214, RUNX1-RUNX1T1, CBFB-MYH11, and gene mutations of KIT, NRAS, KRAS, WT1, NPM1, MLL-PTD, and FLT3-ITD.
[Results]
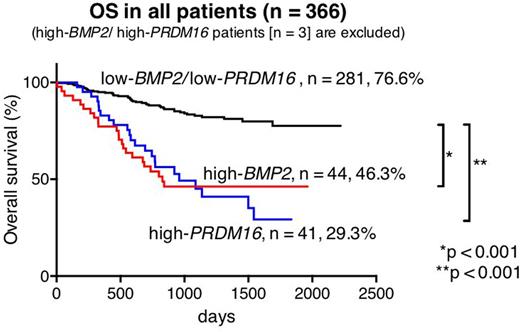
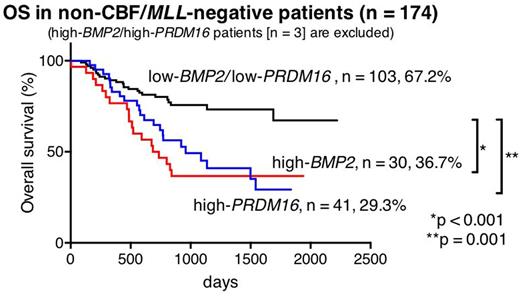
Out of 369 patients, high-BMP2 and high-PRDM16 were identified in 47 (12.7%) and 44 (11.9%) patients, respectively. Only three (0.8%) of 369 patients had both high-BMP2 and high-PRDM16 (high-BMP2/PRDM16). In 44 high-BMP2 patients and 41 high-PRDM16 patients (3 high-BMP2/PRDM16 patients were excluded), MLL rearrangements were only found in high-BMP2 patients (25.5% vs 0%, p < 0.001), whereas FLT3-ITD was significantly much more frequent in high-PRDM16 patients (4.5% vs 56.1%, p < 0.001). Core-Binding-Factor (CBF)-AML was rare in both groups (4.5% and 0%, p = 0.495). Kaplan|Meier analyses revealed that high-BMP2 patients and high-PRDM16 patients had a low 5-year overall survival (OS) (46.3% and 29.3%, respectively). In molecular subgroup analyses, the prognosis of MLL-rearranged patients, including 12 high-BMP2 patients (21.8%), was not influenced by BMP2 expression (5-year OS: high-BMP2 66.7% vs low-BMP2 64.9%, p = 0.488), suggesting that the prognosis of MLL-rearranged patients might primarily be defined by their fusion partner genes and other gene aberrations as previously reported (e.g., EVI1 expression). Considering this result, further survival analyses of the patients lacking CBF-AML or MLL-rearrangements were performed (n = 174, 5-year OS: 50.5%). In this subgroup, 5-year OS of high-BMP2 patients (n = 30) and high-PRDM16 patients (n = 41) were significantly lower than that of low-BMP2/low-PRDM16 patients (36.7% vs 67.2%, p < 0.001, and 29.3% vs 67.2%, p < 0.001, respectively).
[Conclusions]
High-BMP2 and high-PRDM16 could identify distinct subgroups with poor prognosis in pediatric AML. Further investigation of molecular-targeted therapy against these genes may contribute to the improvement of clinical outcome in these subgroups.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.